Mechanical stretch increases Kv1.5 current through an interaction between the S1-S2 linker and N-terminus of the channel
- PMID: 32122972
- PMCID: PMC7136002
- DOI: 10.1074/jbc.RA119.011302
Mechanical stretch increases Kv1.5 current through an interaction between the S1-S2 linker and N-terminus of the channel
Abstract
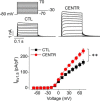
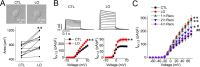
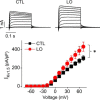
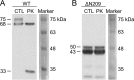
The voltage-gated potassium channel Kv1.5 plays important roles in atrial repolarization and regulation of vascular tone. In the present study, we investigated the effects of mechanical stretch on Kv1.5 channels. We induced mechanical stretch by centrifuging or culturing Kv1.5-expressing HEK 293 cells and neonatal rat ventricular myocytes in low osmolarity (LO) medium and then recorded Kv1.5 current (IKv1.5) in a normal, isotonic solution. We observed that mechanical stretch increased IKv1.5, and this increase required the intact, long, proline-rich extracellular S1-S2 linker of the Kv1.5 channel. The low osmolarity-induced IKv1.5 increase also required an intact intracellular N terminus, which contains the binding motif for endogenous Src tyrosine kinase that constitutively inhibits IKv1.5 Disrupting the Src-binding motif of Kv1.5 through N-terminal truncation or mutagenesis abolished the mechanical stretch-mediated increase in IKv1.5 Our results further showed that the extracellular S1-S2 linker of Kv1.5 communicates with the intracellular N terminus. Although the S1-S2 linker of WT Kv1.5 could be cleaved by extracellularly applied proteinase K (PK), an N-terminal truncation up to amino acid residue 209 altered the conformation of the S1-S2 linker and made it no longer susceptible to proteinase K-mediated cleavage. In summary, the findings of our study indicate that the S1-S2 linker of Kv1.5 represents a mechanosensor that regulates the activity of this channel. By targeting the S1-S2 linker, mechanical stretch may induce a change in the N-terminal conformation of Kv1.5 that relieves Src-mediated tonic channel inhibition and results in an increase in IKv1.5.
Keywords: Kv1.5; Src; Src kinase; cell biology; electrophysiology; ion channel; mechanical stretch; mechanotransduction; molecular biology; patch clamp; potassium channel; structure-function; voltage-gated potassium channel.
© 2020 Milton et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





Similar articles
-
The N terminus and transmembrane segment S1 of Kv1.5 can coassemble with the rest of the channel independently of the S1-S2 linkage.J Biol Chem. 2018 Oct 5;293(40):15347-15358. doi: 10.1074/jbc.RA118.004065. Epub 2018 Aug 17. J Biol Chem. 2018. PMID: 30121572 Free PMC article.
-
Proteolytic cleavage in the S1-S2 linker of the Kv1.5 channel does not affect channel function.Biochim Biophys Acta. 2016 Jun;1858(6):1082-90. doi: 10.1016/j.bbamem.2016.02.012. Epub 2016 Feb 11. Biochim Biophys Acta. 2016. PMID: 26874203
-
Allowed N-glycosylation sites on the Kv1.2 potassium channel S1-S2 linker: implications for linker secondary structure and the glycosylation effect on channel function.Biochem J. 2003 Nov 1;375(Pt 3):769-75. doi: 10.1042/BJ20030517. Biochem J. 2003. PMID: 12911333 Free PMC article.
-
Kv1.5 channels are regulated by PKC-mediated endocytic degradation.J Biol Chem. 2021 Jan-Jun;296:100514. doi: 10.1016/j.jbc.2021.100514. Epub 2021 Mar 4. J Biol Chem. 2021. PMID: 33676894 Free PMC article.
-
Challenges Faced with Small Molecular Modulators of Potassium Current Channel Isoform Kv1.5.Biomolecules. 2019 Dec 19;10(1):10. doi: 10.3390/biom10010010. Biomolecules. 2019. PMID: 31861703 Free PMC article. Review.
Cited by
-
Native American ataxia medicines rescue ataxia-linked mutant potassium channel activity via binding to the voltage sensing domain.Nat Commun. 2023 Jun 6;14(1):3281. doi: 10.1038/s41467-023-38834-6. Nat Commun. 2023. PMID: 37280215 Free PMC article.
-
Meta-Analysis of Mechano-Sensitive Ion Channels in Human Hearts: Chamber- and Disease-Preferential mRNA Expression.Int J Mol Sci. 2023 Jun 30;24(13):10961. doi: 10.3390/ijms241310961. Int J Mol Sci. 2023. PMID: 37446137 Free PMC article.
References
-
- Archer S. L., Souil E., Dinh-Xuan A. T., Schremmer B., Mercier J. C., El Yaagoubi A., Nguyen-Huu L., Reeve H. L., and Hampl V. (1998) Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J. Clin. Invest. 101, 2319–2330 10.1172/JCI333 - DOI - PMC - PubMed
-
- Ohanyan V., Yin L., Bardakjian R., Kolz C., Enrick M., Hakobyan T., Kmetz J., Bratz I., Luli J., Nagane M., Khan N., Hou H., Kuppusamy P., Graham J., Fu F. K., et al. (2015) Requisite role of Kv1.5 channels in coronary metabolic dilation. Circ. Res. 117, 612–621 10.1161/CIRCRESAHA.115.306642 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

