Cortical topological network changes following optic neuritis
- PMID: 32123044
- PMCID: PMC7136064
- DOI: 10.1212/NXI.0000000000000687
Cortical topological network changes following optic neuritis
Abstract
Objective: To differentiate between visual cortical network topology changes following optic neuritis (ON) stemming from different inflammatory disease types, we used mathematical graph theory-based tools to analyze functional imaging data.
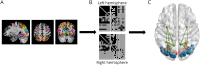
Methods: Sixty-two patients were recruited into this cross-sectional study, 23 of whom had neuromyelitis optica spectrum disorder (NMOSD) with ON, 18 with clinically isolated syndrome (CIS)-ON, and 21 with other CIS episodes. Twenty-six healthy controls (HCs) were also recruited. All participants underwent resting-state functional MRI. Visual networks were defined using 50 visual regions of interest. Analysis included graph theory metrics, including degree, density, modularity, and local and global efficiency.
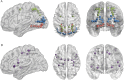
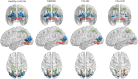
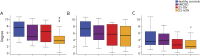
Results: Visual network density shows decreased connectivity in all patient groups compared with controls. A higher degree of connections is seen in both ON groups (CIS and NMOSD) compared with the the non-ON group. This pattern is most pronounced in dorsal-lateral regions. Information transfer efficiency and modularity were reduced in both CIS groups, but not in the NMOSD group, compared with the HC group.
Conclusions: Visual network density appears affected by the neurologic deficit sustained (ON), and connectivity changes are more evident in dorsal-lateral regions. Efficiency and modularity appear to be associated with the specific disease type (CIS vs NMOSD). Thus, topological cortical changes in the visual system are associated with the type of neurologic deficit within the limits set on them by the underlying pathophysiology. We suggest that cortical patterns of activity should be considered in the outcome of the patients despite the localized nature of ON.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

References
-
- Schoonheim MM, Filippi M. Functional plasticity in MS Friend or foe? Neurology 2012;79:1418–1419. - PubMed
-
- Costello F. Vision disturbances in multiple sclerosis. Semin Neurol 2016;36:185–195. - PubMed
-
- Abel A, McClelland C, Lee MS. Critical review: typical and atypical optic neuritis. Surv Ophthalmol 2019;64:770–779. - PubMed
-
- Chen JJ, Pittock SJ, Flanagan EP, Lennon VA, Bhatti MT. Optic neuritis in the era of biomarkers. Surv Ophthalmol 2020;65:12–17. - PubMed
-
- Lennon VA, Wingerchuk DM, Kryzer TJ, et al. . A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106–2112. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
