Active efficient coding explains the development of binocular vision and its failure in amblyopia
- PMID: 32123102
- PMCID: PMC7084066
- DOI: 10.1073/pnas.1908100117
Active efficient coding explains the development of binocular vision and its failure in amblyopia
Abstract
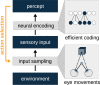
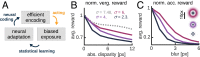
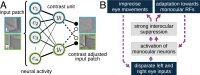
The development of vision during the first months of life is an active process that comprises the learning of appropriate neural representations and the learning of accurate eye movements. While it has long been suspected that the two learning processes are coupled, there is still no widely accepted theoretical framework describing this joint development. Here, we propose a computational model of the development of active binocular vision to fill this gap. The model is based on a formulation of the active efficient coding theory, which proposes that eye movements as well as stimulus encoding are jointly adapted to maximize the overall coding efficiency. Under healthy conditions, the model self-calibrates to perform accurate vergence and accommodation eye movements. It exploits disparity cues to deduce the direction of defocus, which leads to coordinated vergence and accommodation responses. In a simulated anisometropic case, where the refraction power of the two eyes differs, an amblyopia-like state develops in which the foveal region of one eye is suppressed due to inputs from the other eye. After correcting for refractive errors, the model can only reach healthy performance levels if receptive fields are still plastic, in line with findings on a critical period for binocular vision development. Overall, our model offers a unifying conceptual framework for understanding the development of binocular vision.
Keywords: accommodation; active perception; amblyopia; efficient coding; vergence.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


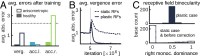
Similar articles
-
The development of active binocular vision under normal and alternate rearing conditions.Elife. 2021 Aug 17;10:e56212. doi: 10.7554/eLife.56212. Elife. 2021. PMID: 34402429 Free PMC article.
-
Interocular velocity cues elicit vergence eye movements in mice.J Neurophysiol. 2020 Aug 1;124(2):623-633. doi: 10.1152/jn.00697.2019. Epub 2020 Jul 29. J Neurophysiol. 2020. PMID: 32727261 Free PMC article.
-
Binocular vision and fixational eye movements.J Vis. 2019 Apr 1;19(4):9. doi: 10.1167/19.4.9. J Vis. 2019. PMID: 30943531
-
The Role of Binocularity in Anisometropic Amblyopia.J Binocul Vis Ocul Motil. 2019 Oct-Dec;69(4):141-152. doi: 10.1080/2576117X.2019.1656034. Epub 2019 Sep 5. J Binocul Vis Ocul Motil. 2019. PMID: 31486743 Review.
-
Binocular vision in amblyopia: structure, suppression and plasticity.Ophthalmic Physiol Opt. 2014 Mar;34(2):146-62. doi: 10.1111/opo.12123. Ophthalmic Physiol Opt. 2014. PMID: 24588532 Review.
Cited by
-
Predictive coding is a consequence of energy efficiency in recurrent neural networks.Patterns (N Y). 2022 Nov 23;3(12):100639. doi: 10.1016/j.patter.2022.100639. eCollection 2022 Dec 9. Patterns (N Y). 2022. PMID: 36569556 Free PMC article.
-
Efficient Temporal Coding in the Early Visual System: Existing Evidence and Future Directions.Front Comput Neurosci. 2022 Jul 4;16:929348. doi: 10.3389/fncom.2022.929348. eCollection 2022. Front Comput Neurosci. 2022. PMID: 35874317 Free PMC article. Review.
-
The development of active binocular vision under normal and alternate rearing conditions.Elife. 2021 Aug 17;10:e56212. doi: 10.7554/eLife.56212. Elife. 2021. PMID: 34402429 Free PMC article.
-
Efficacy of binocular vision training and Fresnel press-on prism on children with esotropia and amblyopia.Int Ophthalmol. 2023 Feb;43(2):583-588. doi: 10.1007/s10792-022-02461-9. Epub 2022 Aug 9. Int Ophthalmol. 2023. PMID: 35945412 Clinical Trial.
-
Sequential Effects in Reaching Reveal Efficient Coding in Motor Planning.bioRxiv [Preprint]. 2024 Dec 10:2024.09.30.615975. doi: 10.1101/2024.09.30.615975. bioRxiv. 2024. PMID: 39416082 Free PMC article. Preprint.
References
-
- Clark D. D., Sokoloff L., “Circulation and energy metabolism of the brain” in Basic Neurochemistry: Molecular, Cellular and Medical Aspects, Siegel G. J., Agranoff B. W., Albers R. W., Fisher S. K., Uhler M. D., Eds. (Lippincott-Raven, Philadelphia, PA, 1999), pp. 637–670.
-
- Barlow H. B., et al. , Possible principles underlying the transformation of sensory messages. Sens. Commun. 1, 217–234 (1961).
-
- Simoncelli E. P., Olshausen B. A., Natural image statistics and neural representation. Annu. Rev. Neurosci. 24, 1193–1216 (2001). - PubMed
-
- Lewicki M. S., Efficient coding of natural sounds. Nat. Neurosci. 5, 356–363 (2002). - PubMed
-
- Atick J. J., Redlich A. N., What does the retina know about natural scenes? Neural Comput. 4, 196–210 (1992).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

