Sex and APOE ε4 genotype modify the Alzheimer's disease serum metabolome
- PMID: 32123170
- PMCID: PMC7052223
- DOI: 10.1038/s41467-020-14959-w
Sex and APOE ε4 genotype modify the Alzheimer's disease serum metabolome
Abstract
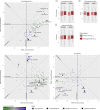
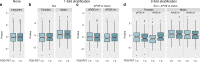
Late-onset Alzheimer's disease (AD) can, in part, be considered a metabolic disease. Besides age, female sex and APOE ε4 genotype represent strong risk factors for AD that also give rise to large metabolic differences. We systematically investigated group-specific metabolic alterations by conducting stratified association analyses of 139 serum metabolites in 1,517 individuals from the AD Neuroimaging Initiative with AD biomarkers. We observed substantial sex differences in effects of 15 metabolites with partially overlapping differences for APOE ε4 status groups. Several group-specific metabolic alterations were not observed in unstratified analyses using sex and APOE ε4 as covariates. Combined stratification revealed further subgroup-specific metabolic effects limited to APOE ε4+ females. The observed metabolic alterations suggest that females experience greater impairment of mitochondrial energy production than males. Dissecting metabolic heterogeneity in AD pathogenesis can therefore enable grading the biomedical relevance for specific pathways within specific subgroups, guiding the way to personalized medicine.
Conflict of interest statement
P.M.D. has received research grants (through Duke University) from Avid/Lilly, Neuronetrix, Avanir, Salix, Alzheimer’s Drug Discovery Foundation, DOD and NIH. P.M.D. has received speaking or advisory fees from Anthrotronix, Neuroptix, Genomind, Clearview, Verily, RBC, Brain Canada, and CEOs Against Alzheimer’s. P.M.D. owns shares in Muses Labs, Anthrotronix, Evidation Health, Turtle Shell Technologies, and Advera Health whose products are not discussed here. P.M.D. served on the board of Baycrest and serves on the board of Apollo Hospitals. P.M.D. is a co-inventor (through Duke) on patents relating to dementia biomarkers, metabolomics, and therapies, which are unlicensed. R.K.D. is inventor on key patents in the field of metabolomics, including applications for Alzheimer disease. M.A., J.B.T., G.K., M.A.M., J.W.T., R.B., X.H., L.S.J.W., A.J.S., K.N. are co-inventors on patent WO2018049268 in this field. J.B.T. further reports investigator-initiated research support from Eli Lilly unrelated to the work reported here. J.Q.T. may accrue revenue in the future on patents submitted by the University of Pennsylvania wherein he is a co-inventor and he received revenue from the sale of Avid to Eli Lilly as a co-inventor on imaging-related patents submitted by the University of Pennsylvania. L.M.S. is a consultant for Eli Lilly, Novartis, and Roche; he provides QC oversight for the Roche Elecsys immunoassay as part of responsibilities for the ADNI3 study. A.J.S. reports investigator-initiated research support from Eli Lilly unrelated to the work reported here. He has received consulting fees and travel expenses from Eli Lilly and Siemens Healthcare and is a consultant to Arkley BioTek. He also receives support from Springer publishing as an editor-in-chief of Brain Imaging and Behavior. M.W.W. reports stock/stock options from Elan, Synarc, travel expenses from Novartis, Tohoku University, Fundacio Ace, Travel eDreams, MCI Group, NSAS, Danone Trading, ANT Congress, NeuroVigil, CHRU-Hopital Roger Salengro, Siemens, AstraZeneca, Geneva University Hospitals, Lilly, University of California, San Diego–ADNI, Paris University, Institut Catala de Neurociencies Aplicades, University of New Mexico School of Medicine, Ipsen, Clinical Trials on Alzheimer’s Disease, Pfizer, AD PD meeting. All other authors declare no competing interests.
Figures
References
-
- Association As. 2017 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2017;13:325–373. doi: 10.1016/j.jalz.2017.02.001. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- R03 AG054936/AG/NIA NIH HHS/United States
- U01 AG061356/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- RF1 AG059093/AG/NIA NIH HHS/United States
- RF1 AG057452/AG/NIA NIH HHS/United States
- RF1 AG057457/AG/NIA NIH HHS/United States
- P01 AG026572/AG/NIA NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
- R01 AG046171/AG/NIA NIH HHS/United States
- U01 AG046152/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- RF1 AG055549/AG/NIA NIH HHS/United States
- R01 LM012535/LM/NLM NIH HHS/United States
- U01 AG061359/AG/NIA NIH HHS/United States
- RF1 AG061872/AG/NIA NIH HHS/United States
- RF1 AG058942/AG/NIA NIH HHS/United States
- RF1 AG051550/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

