Exercise training results in depot-specific adaptations to adipose tissue mitochondrial function
- PMID: 32123205
- PMCID: PMC7052157
- DOI: 10.1038/s41598-020-60286-x
Exercise training results in depot-specific adaptations to adipose tissue mitochondrial function
Abstract
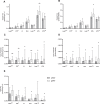
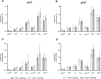
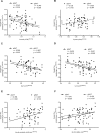
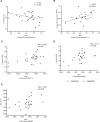
We assessed differences in mitochondrial function in gluteal (gSAT) and abdominal subcutaneous adipose tissue (aSAT) at baseline and in response to 12-weeks of exercise training; and examined depot-specific associations with body fat distribution and insulin sensitivity (SI). Obese, black South African women (n = 45) were randomized into exercise (n = 23) or control (n = 22) groups. Exercise group completed 12-weeks of aerobic and resistance training (n = 20), while the control group (n = 15) continued usual behaviours. Mitochondrial function (high-resolution respirometry and fluorometry) in gSAT and aSAT, SI (frequently sampled intravenous glucose tolerance test), body composition (dual-energy X-ray absorptiometry), and ectopic fat (MRI) were assessed pre- and post-intervention. At baseline, gSAT had higher mitochondrial respiratory capacity and hydrogen peroxide (H2O2) production than aSAT (p < 0.05). Higher gSAT respiration was associated with higher gynoid fat (p < 0.05). Higher gSAT H2O2 production and lower aSAT mitochondrial respiration were independently associated with lower SI (p < 0.05). In response to training, SI improved and gynoid fat decreased (p < 0.05), while H2O2 production reduced in both depots, and mtDNA decreased in gSAT (p < 0.05). Mitochondrial respiration increased in aSAT and correlated with a decrease in body fat and an increase in soleus and hepatic fat content (p < 0.05). This study highlights the importance of understanding the differences in mitochondrial function in multiple SAT depots when investigating the pathophysiology of insulin resistance and associated risk factors such as body fat distribution and ectopic lipid deposition. Furthermore, we highlight the benefits of exercise training in stimulating positive adaptations in mitochondrial function in gluteal and abdominal SAT depots.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

