Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration
- PMID: 32123386
- PMCID: PMC7101073
- DOI: 10.1038/s41591-020-0762-2
Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration
Abstract
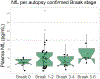
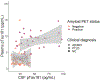
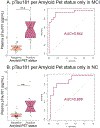
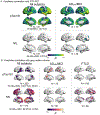
With the potential development of new disease-modifying Alzheimer's disease (AD) therapies, simple, widely available screening tests are needed to identify which individuals, who are experiencing symptoms of cognitive or behavioral decline, should be further evaluated for initiation of treatment. A blood-based test for AD would be a less invasive and less expensive screening tool than the currently approved cerebrospinal fluid or amyloid β positron emission tomography (PET) diagnostic tests. We examined whether plasma tau phosphorylated at residue 181 (pTau181) could differentiate between clinically diagnosed or autopsy-confirmed AD and frontotemporal lobar degeneration. Plasma pTau181 concentrations were increased by 3.5-fold in AD compared to controls and differentiated AD from both clinically diagnosed (receiver operating characteristic area under the curve of 0.894) and autopsy-confirmed frontotemporal lobar degeneration (area under the curve of 0.878). Plasma pTau181 identified individuals who were amyloid β-PET-positive regardless of clinical diagnosis and correlated with cortical tau protein deposition measured by 18F-flortaucipir PET. Plasma pTau181 may be useful to screen for tau pathology associated with AD.
Conflict of interest statement
Competing interest statement:
E.H.T, R.L.J., A.W., A.S., P.W., L.I., V.B., Y.C., H.H., S.S., A.M.K., C.E.T., J.H.K., W.W.S., H.R., B.F.B., and B.L.M. declare no conflict of interest. J.L.D., X.C., N.K.P., D.C.A., S.S., C.D.E., and J.R.S. are employees of Eli Lilly and Company. H.Z. has served at scientific advisory boards for Roche Diagnostics, Wave, Samumed and CogRx, has given lectures in symposia sponsored by Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. K.B. served as a consultant or at advisory boards for Alector, Biogen, CogRx, Lilly, MagQu, Novartis and Roche Diagnostics, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Venture-based platform company at the University of Gothenburg, all unrelated to the work presented in this paper. L.T.G. receives research support from Avid Radiopharmaceuticals, Eli Lilly. She has received consulting fees from the Simon Foundation and Cura Sen, Inc. She serves as associate editor for Frontiers in Aging Neurosciences, Frontiers in Dementia and the Journal of Alzheimer Disease. G.D.R. receives research support from NIH, Alzheimer’s Association, American College of Radiology, Tau Research Consortium, Avid Radiopharmaceuticals, Eli Lilly, GE Healthcare, Life Molecular Imaging. He has served as a consultant for Eisai, Merck, Axon Neurosciences. He received speaking honoraria from GE Healthcare. He serves as Associate Editor for JAMA Neurology. J.C.R. is a site PI for clinical trials supported by Eli Lilly and receives support from NIH. A.L.B. receives research support from NIH, the Tau Research Consortium, the Association for Frontotemporal Degeneration, Bluefield Project to Cure Frontotemporal Dementia, Corticobasal Degeneration Solutions, the Alzheimer’s Drug Discovery Foundation and the Alzheimer's Association. He has served as a consultant for Aeton, Abbvie, Alector, AGTC, Amgen, Arkuda, Arvinas, Asceneuron, Ionis, Lundbeck, Novartis, Passage BIO, Sangamo, Samumed, Third Rock, Toyama and UCB, and received research support from Avid, Biogen, BMS, C2N, Cortice, Eli Lilly, Forum, Genentech, Janssen, Novartis, Pfizer, Roche and TauRx.
Figures







Comment in
-
Another step forward in blood-based diagnostics for Alzheimer's disease.Nat Med. 2020 Mar;26(3):314-316. doi: 10.1038/s41591-020-0797-4. Nat Med. 2020. PMID: 32132715 No abstract available.
-
Closer to a blood test for Alzheimer disease.Nat Rev Neurol. 2020 May;16(5):241. doi: 10.1038/s41582-020-0347-1. Nat Rev Neurol. 2020. PMID: 32203395 No abstract available.
References
-
- Swine flu snipers, Alzheimer’s drug push and Google’s latest gaming bot. Nature 574 602–603 (2019). doi:doi: 10.1038/d41586-019-03266-0 - DOI
Publication types
MeSH terms
Substances
Grants and funding
- P50 AG023501/AG/NIA NIH HHS/United States
- K24 AG045333/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- K24 AG053435/AG/NIA NIH HHS/United States
- R01 AG038791/AG/NIA NIH HHS/United States
- U19 AG063911/AG/NIA NIH HHS/United States
- RF1 AG050967/AG/NIA NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- P01 AG066597/AG/NIA NIH HHS/United States
- R01 MH120794/MH/NIMH NIH HHS/United States
- K23 AG059888/AG/NIA NIH HHS/United States
- K08 AG052648/AG/NIA NIH HHS/United States
- P30 AG062677/AG/NIA NIH HHS/United States
- R01 AG062268/AG/NIA NIH HHS/United States
- U01 AG045390/AG/NIA NIH HHS/United States
- U54 NS092089/NS/NINDS NIH HHS/United States
- R01 AG045611/AG/NIA NIH HHS/United States
- T32 AG023481/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

