Switchable Polymer Materials Controlled by Rotaxane Macromolecular Switches
- PMID: 32123731
- PMCID: PMC7047276
- DOI: 10.1021/acscentsci.0c00002
Switchable Polymer Materials Controlled by Rotaxane Macromolecular Switches
Abstract
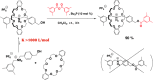
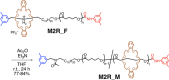
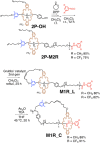
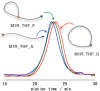
The synthesis and dynamic nature of macromolecular systems controlled by rotaxane macromolecular switches are introduced to discuss the significance of rotaxane linking of polymer chains and its topological switching. Macromolecular switches have been synthesized from macromolecular [2]rotaxanes (M2Rs) using sec-ammonium salt/crown ether couples. The successful synthesis of M2Rs possessing a single polymer axle and one crown ether wheel, constituting a key component of the macromolecular switch, has allowed us to develop various unique applications such as the development of topology-transformable polymers. Polymer topological transformations (e.g., linear-star and linear-cyclic) are achieved using rotaxane-linked polymers and rotaxane macromolecular switches. The pronounced dynamic nature of these polymer systems is sufficiently interesting to design sophisticated stimuli-responsive molecules, polymers, and materials.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The author declares no competing financial interest.
Figures



























References
-
- Switchable and Responsive Surfaces and Materials for Biomedical Applications; Zhang Z., Ed.; Elsevier, 2015. 10.1016/C2013-0-16356-8 - DOI
Publication types
LinkOut - more resources
Full Text Sources

