Prosthesis with neuromorphic multilayered e-dermis perceives touch and pain
- PMID: 32123782
- PMCID: PMC7051004
- DOI: 10.1126/scirobotics.aat3818
Prosthesis with neuromorphic multilayered e-dermis perceives touch and pain
Abstract
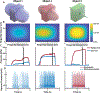
The human body is a template for many state-of-the-art prosthetic devices and sensors. Perceptions of touch and pain are fundamental components of our daily lives that convey valuable information about our environment while also providing an element of protection from damage to our bodies. Advances in prosthesis designs and control mechanisms can aid an amputee's ability to regain lost function but often lack meaningful tactile feedback or perception. Through transcutaneous electrical nerve stimulation (TENS) with an amputee, we discovered and quantified stimulation parameters to elicit innocuous (non-painful) and noxious (painful) tactile perceptions in the phantom hand. Electroencephalography (EEG) activity in somatosensory regions confirms phantom hand activation during stimulation. We invented a multilayered electronic dermis (e-dermis) with properties based on the behavior of mechanoreceptors and nociceptors to provide neuromorphic tactile information to an amputee. Our biologically inspired e-dermis enables a prosthesis and its user to perceive a continuous spectrum from innocuous to noxious touch through a neuromorphic interface that produces receptor-like spiking neural activity. In a Pain Detection Task (PDT), we show the ability of the prosthesis and amputee to differentiate non-painful or painful tactile stimuli using sensory feedback and a pain reflex feedback control system. In this work, an amputee can use perceptions of touch and pain to discriminate object curvature, including sharpness. This work demonstrates possibilities for creating a more natural sensation spanning a range of tactile stimuli for prosthetic hands.
Figures


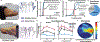

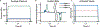
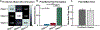

References
-
- Johansson RS, Flanagan JR, Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat. Rev. Neurosci. 10, 345–359 (2009). - PubMed
-
- Vallbo AB, Johansson RS, Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Hum. Neurobiol. 3, 3–14 (1984). - PubMed
-
- Scheibert J, Leurent S, Prevost A, Debregeas G, The role of fingerprints in the coding of tactile information probed with a biomimetic sensor. Science 323, 1503–1506 (2009). - PubMed
-
- Pruszynski JA, Johansson RS, Edge-orientation processing in first-order tactile neurons. Nat. Neurosci. 17, 1404–1409 (2014). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

