A case report of heart transplant for ischaemic cardiomyopathy from lupus coronary vasculitis
- PMID: 32123802
- PMCID: PMC7042145
- DOI: 10.1093/ehjcr/ytz183
A case report of heart transplant for ischaemic cardiomyopathy from lupus coronary vasculitis
Abstract
Background: Coronary vasculitis is a rare, life-threatening complication of systemic lupus erythematosus (SLE).
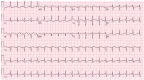
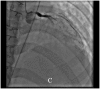
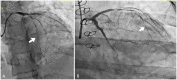
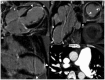
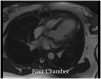
Case summary: A 23-year-old woman with SLE presented with typical angina and worsening dyspnoea on exertion. Coronary angiography revealed severe triple vessel disease with a 'string of beads' appearance classic for coronary vasculitis. Transthoracic echocardiogram revealed ejection fraction of 25-30% with a severely hypokinetic distal septum and distal anterior wall and an akinetic apical wall. Despite vasculitis treatment with cyclophosphamide and pulse-dose steroids, her coronary vasculitis did not improve. She was refractory to anti-anginal and guideline-directed medical therapy for heart failure and successfully underwent orthotopic heart transplant (OHT).
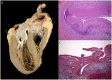
Discussion: This is the first reported case of OHT in the case of SLE coronary vasculitis. Chronic SLE coronary vasculitis is caused by lymphocyic infiltration leading to inflammation and fibrosis of the major epicardial coronary arteries but can be successfully managed with OHT when refractory to medical SLE and heart failure therapies. It can affect patients of all ages with SLE, emphasizing the importance of thorough history taking and clinical evaluation in young patients presenting with cardiac symptoms to establish an appropriate diagnosis and treatment plan.
Keywords: Case report; Coronary vasculitis; Ischaemic cardiomyopathy; Orthotopic heart transplant; Systemic lupus erythematosus.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

Similar articles
-
Lupus-Induced Vasculitis and Multiple Organ Dysfunction Syndrome as the First Presentation of Systemic Lupus Erythematosus (SLE) in Pregnancy.Am J Case Rep. 2020 Apr 14;21:e921299. doi: 10.12659/AJCR.921299. Am J Case Rep. 2020. PMID: 32284523 Free PMC article.
-
Systemic lupus erythematosus with coronary vasculitis and massive myocardial infarction. A case report.S Afr Med J. 1986 Jun 7;69(12):765-8. S Afr Med J. 1986. PMID: 3715654
-
Complementary role of cardiovascular imaging and laboratory indices in early detection of cardiovascular disease in systemic lupus erythematosus.Lupus. 2017 Mar;26(3):227-236. doi: 10.1177/0961203316671810. Epub 2016 Sep 30. Lupus. 2017. PMID: 27687024 Review.
-
Systemic lupus erythematosus coronary vasculitis demonstrated on cardiac computed tomography.Curr Probl Diagn Radiol. 2014 Sep-Oct;43(5):294-7. doi: 10.1067/j.cpradiol.2014.05.005. Curr Probl Diagn Radiol. 2014. PMID: 25088221
-
Successful treatment of gastrointestinal vasculitis due to systemic lupus erythematosus with intravenous pulse cyclophosphamide: a clinical case report and review of the literature.Br J Rheumatol. 1998 Sep;37(9):1023-8. doi: 10.1093/rheumatology/37.9.1023. Br J Rheumatol. 1998. PMID: 9783772 Review.
Cited by
-
Lupus Vasculitis: An Overview.Biomedicines. 2021 Nov 5;9(11):1626. doi: 10.3390/biomedicines9111626. Biomedicines. 2021. PMID: 34829857 Free PMC article. Review.
References
-
- Caracciolo EA, Marcu CB, Ghantous A, Donohue TJ, Hutchinson G.. Coronary vasculitis with acute myocardial infarction in a young woman with systemic lupus erythematosus. J Clin Rheumatol 2004;10:66.. - PubMed
-
- Matayoshi AH, Dhond MR, Laslett LJ.. Multiple coronary aneurysms in a case of systemic lupus erythematosus. Chest 1999;116:1116–1118. - PubMed
-
- Heibel RH, O'Toole JD, Curtiss EI, Medsger TA, Reddy SP, Shaver JA.. Coronary arteritis in systemic lupus erythematosus. Chest 1976;69:700–703. - PubMed
-
- Bonfiglio TA, Botti RE, Hagstrom J.. Coronary arteritis, occlusion, and myocardial infarction due to lupus erythematosus. Am Heart J 1972;83:153–158. - PubMed
-
- M. Korbet S, M. Schwartz M, J. Lewis E.. Immune complex deposition and coronary vasculitis in systemic lupus erythematosus: report of two cases. Am J Med 1984;77:141–146. - PubMed
LinkOut - more resources
Full Text Sources
