Clinical translation of 18F-fluoropivalate - a PET tracer for imaging short-chain fatty acid metabolism: safety, biodistribution, and dosimetry in fed and fasted healthy volunteers
- PMID: 32123971
- PMCID: PMC7515955
- DOI: 10.1007/s00259-020-04724-y
Clinical translation of 18F-fluoropivalate - a PET tracer for imaging short-chain fatty acid metabolism: safety, biodistribution, and dosimetry in fed and fasted healthy volunteers
Abstract
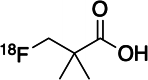
Background: Fatty acids derived de novo or taken up from the extracellular space are an essential source of nutrient for cell growth and proliferation. Radiopharmaceuticals including 11C-acetate, and 18F-FAC (2-18F-fluoroacetate), have previously been used to study short-chain fatty acid (SCFA) metabolism. We developed 18F-fluoropivalate (18F-FPIA; 3-18F-fluoro-2,2-dimethylpropionic acid) bearing a gem-dimethyl substituent to assert metabolic stability for studying SCFA metabolism. We report the safety, biodistribution, and internal radiation dosimetry profile of 18F-FPIA in 24 healthy volunteers and the effect of dietary conditions.
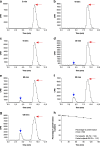
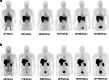
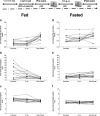
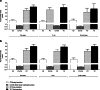
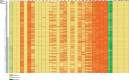
Materials and methods: Healthy volunteer male and female subjects were enrolled (n = 24), and grouped into 12 fed and 12 fasted. Non-esterified fatty acids (NEFA) and carnitine blood measurements were assessed. Subjects received 159.48 MBq (range, 47.31-164.66 MBq) of 18F-FPIA. Radiochemical purity was > 99%. Safety data were obtained during and 24 h after radiotracer administration. Subjects underwent detailed multiple whole-body PET/CT scanning with sampling of venous bloods for radioactivity and radioactive metabolite quantification. Regions of interest were defined to derive individual and mean organ residence times; effective dose was calculated using OLINDA 1.1.
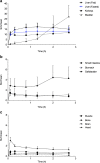
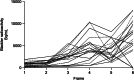
Results: All subjects tolerated 18F-FPIA with no adverse events. Over 90% of radiotracer was present in plasma at 60 min post-injection. The organs receiving highest absorbed dose (in mGy/MBq) were the liver (0.070 ± 0.023), kidneys (0.043 ± 0.013), gallbladder wall (0.026 ± 0.003), and urinary bladder (0.021 ± 0.004); otherwise there was low tissue uptake. The calculated effective dose using mean organ residence times over all 24 subjects was 0.0154 mSv/MBq (SD ± 0.0010). No differences in biodistribution or dosimetry were seen in fed and fasted subjects, though systemic NEFA and carnitine levels reflected fasted and fed states.
Conclusion: The favourable safety, imaging, and dosimetric profile makes 18F-FPIA a promising candidate radiotracer for tracing SCFA metabolism.
Keywords: 18F-FPIA; 18F-Fluoropivalate; Carnitine; Dosimetry; Positron emission tomography; Short chain fatty acid metabolism.
Conflict of interest statement
S Dubash declares no conflicts of interest. N Keat declares no conflicts of interest. K. Kozlowski declares no conflicts of interest. L Allott declares no conflicts of interest. C Barnes declares no conflicts of interest. D Brickute declares no conflicts of interest. S Hill declares no conflicts of interest. M Huiban declares no conflicts of interest. T Barwick declares no conflicts of interest. L Kenny declares no conflicts. E Aboagye is an inventor on PCT/GB2014/051405.
Figures
Comment in
-
Feasibility of [18F]fluoropivalate hybrid PET/MRI for imaging lower and higher grade glioma: a prospective first-in-patient pilot study.Eur J Nucl Med Mol Imaging. 2023 Nov;50(13):3982-3995. doi: 10.1007/s00259-023-06330-0. Epub 2023 Jul 25. Eur J Nucl Med Mol Imaging. 2023. PMID: 37490079 Free PMC article.
References
-
- Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11(5):325–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

