Breast cancer outcome in relation to bone mineral density and bisphosphonate use: a sub-study of the DATA trial
- PMID: 32124136
- PMCID: PMC7103013
- DOI: 10.1007/s10549-020-05567-9
Breast cancer outcome in relation to bone mineral density and bisphosphonate use: a sub-study of the DATA trial
Abstract
Purpose: The phase III DATA study compared 6 and 3 years of adjuvant anastrozole following 2-3 years of tamoxifen in postmenopausal breast cancer patients. This pre-planned side-study assessed the relationship between a reduced bone mineral density (BMD) and distant recurrence-free survival (DRFS), and evaluated the effect of bisphosphonates on DRFS.
Methods: We selected all patients with a BMD measurement within 3 years after randomisation (landmark) without any DRFS events. Kaplan-Meier methods and Cox proportional hazards models were used for analyses.
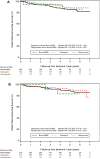
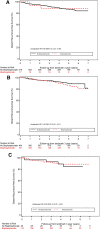
Results: Of 1860 eligible patients, 1142 had a DEXA scan before the landmark. The BMD was normal in 436 (38.2%) and showed osteopenia in 565 (49.5%) and osteoporosis in 141 (12.3%) patients. After a median follow-up of 5.0 years from the landmark, neither osteopenia nor osteoporosis (compared with normal BMD) were associated with DRFS in both the 6-year [osteopenia HR 0.82 (95% CI 0.45-1.49), osteoporosis HR 1.10 (95% CI 0.26-4.67)] and the 3-year arm [osteopenia HR 0.75 (95% CI 0.40-1.42), osteoporosis HR 1.86 (95% CI 0.43-8.01)]. Moreover, bisphosphonate use did not impact DRFS.
Conclusion: No association was observed between a reduced BMD and DRFS. Neither did we observe an impact of bisphosphonates on DRFS.
Keywords: Aromatase inhibitor; Bisphosphonates; Bone health; Bone metastases; Breast cancer; Distant recurrence-free survival; Osteoporosis; Survival; Tamoxifen.
Conflict of interest statement
IEGvH, SCL and ACPS report institutional grants from AstraZeneca during the conduct of the study. MdB reports research grants from Roche, Eisai, Novartis, Pfizer, and Lily during the conduct of the study; and consultation/speakers fee from Roche, Novartis, and Pfizer. VCGT-H reports consultant/advisory role to Pfizer, Lily, and Novartis, and grants to the institute for research from AstraZeneca, Roche, Novartis, Pfizer, and Lilly during the conduct of the study. FWPJvdB, FLGE, HdG, AH, ALTI, JRK, PGMP, WKdR, MJCvdS, CMS, CHS declare no conflicts of interests.
Figures

References
-
- Paterson AH, Anderson SJ, Lembersky BC, et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012;13:734–742. doi: 10.1016/S1470-2045(12)70226-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

