Up-regulation of indoleamine 2,3-dioxygenase 1 (IDO1) expression and catalytic activity is associated with immunosuppression and poor prognosis in penile squamous cell carcinoma patients
- PMID: 32125093
- PMCID: PMC7163927
- DOI: 10.1002/cac2.12001
Up-regulation of indoleamine 2,3-dioxygenase 1 (IDO1) expression and catalytic activity is associated with immunosuppression and poor prognosis in penile squamous cell carcinoma patients
Abstract
Background: Indoleamine 2,3-dioxygenase 1 (IDO1) and tryptophan (Trp) catabolism have been demonstrated to play an important role in tumor immunosuppression. This study examined the expression and catalytic activity of IDO1 in penile squamous cell carcinoma (PSCC) and explored their clinical significance.
Methods: IDO1 expression level, serum concentrations of Trp and kynurenine (Kyn) were examined in 114 PSCC patients by immunohistonchemistry and solid-phase extraction-liquid chromatography-tandem mass spectrometry. The survival was analyzed using Kaplan-Meier method and the log-rank test. Hazard ratio of death was analyzed via univariate and multivariate Cox regression. Immune cell types were defined by principal component analysis. The correlativity was assessed by Pearson's correlation analysis.
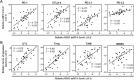
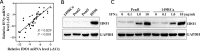
Results: The expression level of IDO1 in PSCC cells was positively correlated with serum Kyn concentration and Kyn/Trp radio (KTR; both P < 0.001) but negatively correlated with serum Trp concentration (P = 0.001). Additionally, IDO1 up-regulation in cancer cells and the increase of serum KTR were significantly associated with advanced N stage (both P < 0.001) and high pathologic grade (P = 0.008 and 0.032, respectively). High expression level of IDO1 in cancer cells and serum KTR were associated with short disease-specific survival (both P < 0.001). However, besides N stage (hazard radio [HR], 6.926; 95% confidence interval [CI], 2.458-19.068; P < 0.001) and pathologic grade (HR, 2.194; 95% CI, 1.021-4.529; P = 0.038), only serum KTR (HR, 2.780; 95% CI, 1.066-7.215; P = 0.036) was an independent predictor for PSCC prognosis. IDO1 expression was positively correlated with the expression of interferon-γ (IFNγ, P < 0.001) and immunosuppressive markers (programmed cell death protein 1, cytotoxic T-lymphocyte-associated protein 4 and programmed death-ligand 1 and 2; all P < 0.05), and the infiltration of immune cells (including cytotoxic T lymphocytes, regulatory T lymphocytes, tumor-associated macrophages, and myeloid-derived suppressor cells; all P < 0.001) in PSCC tissues. Furthermore, the expression of IDO1 was induced by IFNγ in a dose-dependent manner in PSCC cells.
Conclusions: IFNγ-induced IDO1 plays a crucial role in immunoediting and immunosuppression in PSCC. Additionally, serum KTR, an indicator of IDO1 catabolic activity, can be utilized as an independent prognostic factor for PSCC.
Keywords: cytotoxic T-lymphocyte-associated protein 4; immunosuppression; indoleamine 2,3-dioxygenase 1; interferon-gamma; kynurenine/tryptophan ratio; penile cancer; programmed cell death protein 1; programmed death-ligand 1; tumor-infiltrating immune cells.
© 2020 The Authors. Cancer Communications published by John Wiley & Sons Australia, Ltd. on behalf of Sun Yat-sen University Cancer Center.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

