Clinical expert consensus document on quantitative coronary angiography from the Japanese Association of Cardiovascular Intervention and Therapeutics
- PMID: 32125622
- PMCID: PMC7105443
- DOI: 10.1007/s12928-020-00653-7
Clinical expert consensus document on quantitative coronary angiography from the Japanese Association of Cardiovascular Intervention and Therapeutics
Abstract
Quantitative coronary angiography (QCA) remains to play an important role in clinical trials and post-marketing surveillance related to the safety and efficacy of new PCI devices. In this document, the current standard methodology of QCA is summarized. In addition, its history, recent development and future perspectives are also reviewed.
Keywords: Coronary artery disease; Coronary artery stents; Expert consensus document; Quantitative coronary angiography.
Conflict of interest statement
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures


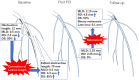



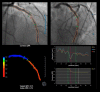
Similar articles
-
Clinical use of physiological lesion assessment using pressure guidewires: an expert consensus document of the Japanese Association of Cardiovascular Intervention and Therapeutics.Cardiovasc Interv Ther. 2019 Jan;34(1):85-96. doi: 10.1007/s12928-018-0559-0. Epub 2018 Dec 27. Cardiovasc Interv Ther. 2019. PMID: 30588572 Review.
-
Consensus document on the clinical application of invasive functional coronary angiography from the Japanese Association of Cardiovascular Intervention and Therapeutics.Cardiovasc Interv Ther. 2024 Apr;39(2):109-125. doi: 10.1007/s12928-024-00988-5. Epub 2024 Feb 17. Cardiovasc Interv Ther. 2024. PMID: 38367157 Free PMC article. Review.
-
Fractional flow reserve calculation from 3-dimensional quantitative coronary angiography and TIMI frame count: a fast computer model to quantify the functional significance of moderately obstructed coronary arteries.JACC Cardiovasc Interv. 2014 Jul;7(7):768-77. doi: 10.1016/j.jcin.2014.03.004. JACC Cardiovasc Interv. 2014. PMID: 25060020
-
Clinical use of physiological lesion assessment using pressure guidewires: an expert consensus document of the Japanese association of cardiovascular intervention and therapeutics-update 2022.Cardiovasc Interv Ther. 2022 Jul;37(3):425-439. doi: 10.1007/s12928-022-00863-1. Epub 2022 May 11. Cardiovasc Interv Ther. 2022. PMID: 35543896 Review.
-
Anatomical and functional assessment of Tryton bifurcation stent before and after final kissing balloon dilatation: Evaluations by three-dimensional coronary angiography, optical coherence tomography imaging and fractional flow reserve.Catheter Cardiovasc Interv. 2017 Jul;90(1):E1-E10. doi: 10.1002/ccd.26777. Epub 2016 Aug 27. Catheter Cardiovasc Interv. 2017. PMID: 27567002
Cited by
-
In-hospital mortality among consecutive patients with ST-Elevation myocardial infarction in modern primary percutaneous intervention era ~ Insights from 15-year data of single-center hospital-based registry ~.PLoS One. 2021 Jun 11;16(6):e0252503. doi: 10.1371/journal.pone.0252503. eCollection 2021. PLoS One. 2021. PMID: 34115767 Free PMC article.
-
Relationship between stress hyperglycemia ratio and progression of non target coronary lesions: a retrospective cohort study.Diabetol Metab Syndr. 2025 Jan 22;17(1):27. doi: 10.1186/s13098-024-01575-7. Diabetol Metab Syndr. 2025. PMID: 39844266 Free PMC article.
-
Relation of GRACE Risk Score to Coronary Lipid Core Plaques in Patients with Acute Coronary Syndrome.Life (Basel). 2023 Feb 24;13(3):630. doi: 10.3390/life13030630. Life (Basel). 2023. PMID: 36983786 Free PMC article.
-
The Application of Deep Learning for the Segmentation and Classification of Coronary Arteries.Diagnostics (Basel). 2023 Jul 5;13(13):2274. doi: 10.3390/diagnostics13132274. Diagnostics (Basel). 2023. PMID: 37443668 Free PMC article.
-
Clinical and procedure characteristics in patients treated with polytetrafluoroethylene-covered stents after coronary perforation: a CIRC-8U multicenter registry and literature review.Cardiovasc Interv Ther. 2021 Oct;36(4):418-428. doi: 10.1007/s12928-020-00716-9. Epub 2020 Oct 9. Cardiovasc Interv Ther. 2021. PMID: 33037569 Review.
References
-
- Byrne RA, Serruys PW, Baumbach A, Escaned J, Fajadet J, James S, et al. Report of a European Society of Cardiology-European Association of Percutaneous Cardiovascular Interventions task force on the evaluation of coronary stents in Europe: executive summary. Eur Heart J. 2015;36:2608–2620. - PubMed
-
- Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, et al. Standardized end point definitions for coronary intervention trials: the Academic Research Consortium-2 Consensus Document. Circulation. 2018;137:2635–2650. - PubMed
-
- Farb A, Zuckerman BD. Clinical event adjudication in cardiovascular device trials: an Food and Drug Administration perspective. Am Heart J. 2017;191:62–64. - PubMed
-
- West JW, Guzman SV. Coronary dilatation and constriction visualized by selective arteriography. Circ Res. 1959;7:527–536. - PubMed
-
- Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51:5–40. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

