Design of the SILICOFCM study: Effect of sacubitril/valsartan vs lifestyle intervention on functional capacity in patients with hypertrophic cardiomyopathy
- PMID: 32125709
- PMCID: PMC7244301
- DOI: 10.1002/clc.23346
Design of the SILICOFCM study: Effect of sacubitril/valsartan vs lifestyle intervention on functional capacity in patients with hypertrophic cardiomyopathy
Abstract
Background: Hypertrophic cardiomyopathy (HCM) is the most common genetic cardiovascular disease with a broad spectrum of disease severity. HCM ranges from a benign course to a progressive disorder characterized by angina, heart failure, malignant arrhythmia, syncope, or sudden cardiac death. So far, no medical treatment has reliably shown to halt or reverse progression of HCM or to alleviate its symptoms. While the angiotensin receptor neprilysin inhibitor sacubitril/valsartan has shown to reduce mortality and hospitalization in heart failure with reduced ejection fraction, data on its effect on HCM are sparse.
Hypothesis: A 4-month pharmacological (sacubitril/valsartan) or lifestyle intervention will significantly improve exercise tolerance (ie, peak oxygen consumption) in patients with nonobstructive HCM compared to the optimal standard therapy (control group).
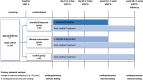
Methods: SILICOFCM is a prospective, multicenter, open-label, randomized, controlled, three-arm clinical trial (NCT03832660) that will recruit 240 adult patients with a confirmed diagnosis of nonobstructive HCM. Eligible patients are randomized to sacubitril/valsartan, lifestyle intervention (physical activity and dietary supplementation with inorganic nitrate), or optimal standard therapy alone (control group). The primary endpoint is the change in functional capacity (ie, peak oxygen consumption). Secondary endpoints include: (a) Change in cardiac structure and function as assessed by transthoracic echocardiography and cardiac magnetic resonance (MRI imaging), (b) change in biomarkers (ie, CK, CKMB, and NT-proBNP), (c) physical activity, and (d) quality of life.
Results: Until December 2019, a total of 41 patients were recruited into the ongoing SILICOFCM study and were allocated to the study groups and the control group. There was no significant difference in key baseline characteristics between the three groups.
Conclusion: The SILICOFCM study will provide novel evidence about the effect of sacubitril/valsartan or lifestyle intervention on functional capacity, clinical phenotype, injury and stretch activation markers, physical activity, and quality of life in patients with nonobstructive HCM.
Keywords: HCM; familial cardiomyopathy; hereditary cardiac disease; left ventricular hypertrophy.
© 2020 The Authors. Clinical Cardiology published by Wiley Periodicals, Inc.
Conflict of interest statement
This trial has received funding from the European Union's Horizon 2020 Research and Innovation Programme under Grant Agreement no. 777204.
References
-
- Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381:242‐255. - PubMed
-
- Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379:1977. - PubMed
-
- Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:2761‐2796. - PubMed
-
- Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. 2014;64:83‐99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials