Characterization of the IgA response to PRRS virus in pig oral fluids
- PMID: 32126095
- PMCID: PMC7053757
- DOI: 10.1371/journal.pone.0229065
Characterization of the IgA response to PRRS virus in pig oral fluids
Abstract
Porcine Reproductive and Respiratory Syndrome (PRRS) is a complex model of host/virus relationship. Disease control measures often includes "acclimatization", i.e. the exposure of PRRS-naïve gilts and sows to PRRSV-infected pigs and premises before the breeding period. In this respect, we had repeatedly observed an association between PRRSV-specific IgA responses in oral fluids (OF) of gilts and block of PRRSV spread. Therefore, we set out to investigate in vitro the inhibition of PRRSV replication by OF samples with different titers of PRRSV-specific IgA and IgG antibody, using Real-time RT PCR. PRRSV yield reduction in monocyte-derived macrophages was associated with the IgA content in OF samples, whereas the IgG-rich samples were sometimes associated with antibody-dependent enhancement (ADE) of replication. Accordingly, we could discriminate between ADE-positive and ADE-negative PRRSV strains. Next, we separated Ig isotypes in OF samples of PRRSV-infected pigs by means of protein A and size exclusion chromatography. The above results were confirmed by using separated Ig isotypes. Both dimeric and monomeric IgA were associated with the strongest reduction of PRRSV replication. The treatment of pig macrophages with separated OF antibodies before PRRSV infection was also associated with PRRSV yield reduction, along with clear changes of both CD163 and CD169 surface expression. Our results point at a role of mucosal IgA in the control of PRRSV replication by extra- and/or intracellular interaction with PRRSV, as well as by induction of signals leading to a reduced susceptibility of macrophages to PRRSV infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


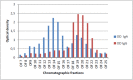
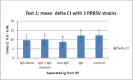


Similar articles
-
Comparative evaluation of immune responses of swine in PRRS-stable and unstable herds.Vet Immunol Immunopathol. 2018 Jun;200:32-39. doi: 10.1016/j.vetimm.2018.04.007. Epub 2018 Apr 22. Vet Immunol Immunopathol. 2018. PMID: 29776610
-
Detection of porcine reproductive and respiratory syndrome virus (PRRSV)-specific IgM-IgA in oral fluid samples reveals PRRSV infection in the presence of maternal antibody.Vet Microbiol. 2018 Feb;214:13-20. doi: 10.1016/j.vetmic.2017.11.011. Epub 2017 Nov 17. Vet Microbiol. 2018. PMID: 29408024
-
Development and validation of an assay to detect porcine reproductive and respiratory syndrome virus-specific neutralizing antibody titers in pig oral fluid samples.Clin Vaccine Immunol. 2013 Aug;20(8):1305-13. doi: 10.1128/CVI.00276-13. Epub 2013 Jun 19. Clin Vaccine Immunol. 2013. PMID: 23784856 Free PMC article.
-
Immunological responses of swine to porcine reproductive and respiratory syndrome virus infection.Viral Immunol. 2002;15(4):533-47. doi: 10.1089/088282402320914485. Viral Immunol. 2002. PMID: 12513925 Review.
-
Role of genetic factors in different swine breeds exhibiting varying levels of resistance/susceptibility to PRRSV.Virus Res. 2023 Mar;326:199057. doi: 10.1016/j.virusres.2023.199057. Epub 2023 Feb 3. Virus Res. 2023. PMID: 36731630 Free PMC article. Review.
Cited by
-
Artificial Intelligence for Automatic Monitoring of Respiratory Health Conditions in Smart Swine Farming.Animals (Basel). 2023 Jun 2;13(11):1860. doi: 10.3390/ani13111860. Animals (Basel). 2023. PMID: 37889795 Free PMC article. Review.
-
Combined Subcutaneous-Intranasal Immunization With Epitope-Based Antigens Elicits Binding and Neutralizing Antibody Responses in Serum and Mucosae Against PRRSV-2 and SARS-CoV-2.Front Immunol. 2022 Mar 31;13:848054. doi: 10.3389/fimmu.2022.848054. eCollection 2022. Front Immunol. 2022. PMID: 35432364 Free PMC article.
-
Effect of vaccination route (intradermal vs. intramuscular) against porcine reproductive and respiratory syndrome using a modified live vaccine on systemic and mucosal immune response and virus transmission in pigs.BMC Vet Res. 2024 Jan 3;20(1):5. doi: 10.1186/s12917-023-03853-4. BMC Vet Res. 2024. PMID: 38172908 Free PMC article.
-
Porcine respiratory disease complex: Dynamics of polymicrobial infections and management strategies after the introduction of the African swine fever.Front Vet Sci. 2022 Nov 25;9:1048861. doi: 10.3389/fvets.2022.1048861. eCollection 2022. Front Vet Sci. 2022. PMID: 36504860 Free PMC article. Review.
-
Testable Candidate Immune Correlates of Protection for Porcine Reproductive and Respiratory Syndrome Virus Vaccination.Vaccines (Basel). 2023 Mar 5;11(3):594. doi: 10.3390/vaccines11030594. Vaccines (Basel). 2023. PMID: 36992179 Free PMC article. Review.
References
-
- Zimmerman J, Benfield DA, Murtaugh MP, Osorio F, Stevenson GW, Tottemorell M. Porcine reproductive and Respiratory Syndrome Virus (Porcine Arterivirus). 2006:387–417.
-
- Holtkamp DJ, Polson DD, Torremorell M, Morrison B, Classen DM, Becton L, et al. Terminology for classifying swine herds by porcine reproductive and respiratory syndrome virus status. Tierarztl Prax Ausg G Grosstiere Nutztiere 2011;39(2):101–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

