Fingolimod after a first unilateral episode of acute optic neuritis (MOVING) - preliminary results from a randomized, rater-blind, active-controlled, phase 2 trial
- PMID: 32126977
- PMCID: PMC7052969
- DOI: 10.1186/s12883-020-01645-z
Fingolimod after a first unilateral episode of acute optic neuritis (MOVING) - preliminary results from a randomized, rater-blind, active-controlled, phase 2 trial
Abstract
Background: Neuroprotection and promotion of remyelination represent important therapeutic gaps in multiple sclerosis (MS). Acute optic neuritis (ON) is a frequent MS manifestation. Based on the presence and properties of sphingosine-1-phosphate receptors (S1PR) on astrocytes and oligodendrocytes, we hypothesized that remyelination can be enhanced by treatment with fingolimod, a S1PR modulator currently licensed for relapsing-remitting MS.
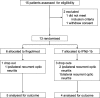
Methods: MOVING was an investigator-driven, rater-blind, randomized clinical trial. Patients with acute unilateral ON, occurring as a clinically isolated syndrome or MS relapse, were randomized to 6 months of treatment with 0.5 mg oral fingolimod or subcutaneous IFN-β 1b 250 μg every other day. The change in multifocal visual evoked potential (mfVEP) latency of the qualifying eye was examined as the primary (month 6 vs. baseline) and secondary (months 3, 6 and 12 vs. baseline) outcome. In addition, full field visual evoked potentials, visual acuity, optical coherence tomography as well as clinical relapses and measures of disability, cerebral MRI, and self-reported visual quality of life were obtained for follow-up. The study was halted due to insufficient recruitment (n = 15), and available results are reported.
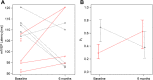
Results: Per protocol analysis of the primary endpoint revealed a significantly larger reduction of mfVEP latency at 6 months compared to baseline with fingolimod treatment (n = 5; median decrease, 15.7 ms) than with IFN-β 1b treatment (n = 4; median increase, 8.15 ms) (p < 0.001 for interaction). Statistical significance was maintained in the secondary endpoint analysis. Descriptive results are reported for other endpoints.
Conclusion: Preliminary results of the MOVING trial argue in support of a beneficial effect of fingolimod on optic nerve remyelination when compared to IFN-β treatment. Interpretation is limited by the small number of complete observations, an unexpected deterioration of the control group and a difference in baseline mfVEP latencies. The findings need to be confirmed in larger studies.
Trial registration: The trial was registered as EUDRA-CT 2011-004787-30 on October 26, 2012 and as NCT01647880 on July 24, 2012.
Keywords: Fingolimod; Interferon Beta-1b; Multifocal VEP; Optic neuritis; Remyelination.
Conflict of interest statement
CA has received honoraria from Biogen, Genzyme, Merck and Novartis and travel support from Almirall, Biogen, Merck and Teva. JM has received speaking fees from Teva, Biogen and Novartis, unrelated to this study. HGZ has received speaking fees from Teva and research grants from Novartis, unrelated to this study. JD has received grants by Bayer and Novartis; honoraria from Bayer, Biogen, Genzyme, Merck, Novartis and Roche; travel support from Bayer, Biogen, Merck and Novartis. JBS has received travel grants and speaking fees from Bayer Healthcare, Biogen Idec, Merck Serono, Sanofi-aventis/Genzyme, and Teva. AUB is cofounder and shareholder of technology startups Motognosis GmbH and Nocturne GmbH. He is named as inventor on several patent applications describing serum biomarkers for multiple sclerosis, perceptive computing for motor symptoms and retinal image analysis using optical coherence tomography. FP has received honoraria and research support from Alexion, Bayer, Biogen, Chugai, Merck-Serono, Novartis, Genzyme, MedImmune, Shire, Teva, and serves on scientific advisory boards for Alexion, MedImmune and Novartis. He has received funding from Deutsche Forschungsgemeinschaft (DFG Exc 257), Bundesministerium für Bildung und Forschung (Competence Network Multiple Sclerosis), Guthy Jackson Charitable Foundation, EU Framework Program 7, National Multiple Sclerosis Society of the USA. OH has received honoraria, travel and/or research grants from Alexion, Bayer, Biogen, Boehringer Ingelheim, Celgene, Daiichi-Sankyo, Merck, Novartis, Roche, Sanofi and Teva. AL, CN, SKP, MS and CC have nothing to disclose.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

