Safety and efficacy of a standardized intracameral combination of mydriatics and anesthetic for cataract surgery in type-2 diabetic patients
- PMID: 32126990
- PMCID: PMC7055021
- DOI: 10.1186/s12886-020-01343-x
Safety and efficacy of a standardized intracameral combination of mydriatics and anesthetic for cataract surgery in type-2 diabetic patients
Abstract
Background: Cataract surgery in diabetics is more technically challenging due to a number of factors including poor intraoperative pupil dilation and a higher risk of vision threatening complications. This study evaluates the safety and efficacy of an intracameral combination of 2 mydriatics and 1 anesthetic (ICMA, Mydrane) for cataract surgery in patients with well-controlled type-2 diabetes.
Methods: Post-hoc subgroup analysis of a phase 3 randomized study, comparing ICMA to a conventional topical regimen. Data were collected from 68 centers in Europe and Algeria. Only well-controlled type-2 diabetics, free of pre-proliferative retinopathy, were included. The results for non-diabetics are also reported. The primary efficacy variable was successful capsulorhexis without additional mydriatic treatment. Postoperative safety included adverse events, endothelial cell density and vision.
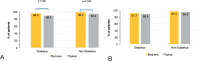
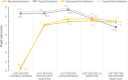
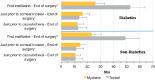
Results: Among 591 randomized patients, 57 (9.6%) had controlled type 2 diabetes [24 (42.1%) in the ICMA Group and 33 (57.9%) in the Topical Group; intention-to-treat (ITT) set]. Among diabetics, capsulorhexis was successfully performed without additional mydriatics in 24 (96.0%; modified-ITT set) patients in the ICMA Group and 26 (89.7%) in the Topical Group. These proportions were similar in non-diabetics. No diabetic patient [1 (0.5%) non-diabetics] in the ICMA Group had a significant decrease in pupil size (≥3 mm) intraoperatively compared to 4 (16.0%; modified-ITT set) diabetics [16 (7.3%) non-diabetics] in the Topical group. Ocular AE among diabetics occurred in 2 (8.0%; Safety set) patients in the ICMA Group and 5 (16.7%) in the Topical Group. Endothelial cell density at 1 month postoperatively was similar between groups in diabetics (P = 0.627) and non-diabetics (P = 0.368).
Conclusions: ICMA is effective and can be safely used in patients with well-controlled diabetes, with potential advantages compared to a topical regimen including reduced systemic risk, better corneal integrity and reduced risk of ocular complications.
Trial registration: The trial was registered at (reference # NCT02101359) on April 2, 2014.
Keywords: Anesthetics; Cataract surgery; Diabetes; Intracameral; Mydrane; Mydriatics.
Conflict of interest statement
Dr. Labetoulle reports personal fees from ALCON, personal fees from Allergan, personal fees from Bausch & Lomb, personal fees from Dompe, personal fees from Horus, personal fees from MSD, personal fees from Novartis, personal fees from Santen, personal fees from Shire, grants and personal fees from Laboratoires Thea, outside the submitted work. Dr. Behndig has nothing to report. Dr. Tassignon reports personal fees from Laboratoires Thea, personal fees from Zeiss, personal fees from Morcher, personal fees from Ziemer, outside the submitted work. Dr. Nuijts reports grants, personal fees and non-financial support from Laboratoires Thea, during the conduct of the study; grants from Alcon, grants from Abbott AMO, grants from Bausch and Lomb, outside the submitted work. Dr. Mencucci has nothing to disclose. Dr. Pleyer has nothing to disclose. Dr. Güell is a Consultant for Ophtec, Kowa Zeiss Meditec, Alcon, Laboratoires Thea, Orca Surgical and an owner of Calhoun Vision and Visiometrics and received a grant from instituto Microcirugia Ocular. Dr. Szaflik has nothing to disclose. Dr. Rosen has nothing to disclose. Dr. Bérard has received funding for participating in the study from Laboratoires Thea. Dr. Chiambaretta was a consultant for Laboratoires Thea, Novartis and Santen. Dr. Cochener-Lamard reports grants from Alcon, personal fees from Zeiss, personal fees from Johnson and Johnson, personal fees from Hoya, grants and personal fees from Laboratoires Thea, personal fees from Santen, personal fees from Cutting Edge, and personal fees from Horus, outside the submitted work.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

