Heterosubtypic Protection Induced by a Live Attenuated Influenza Virus Vaccine Expressing Galactose-α-1,3-Galactose Epitopes in Infected Cells
- PMID: 32127444
- PMCID: PMC7064743
- DOI: 10.1128/mBio.00027-20
Heterosubtypic Protection Induced by a Live Attenuated Influenza Virus Vaccine Expressing Galactose-α-1,3-Galactose Epitopes in Infected Cells
Abstract
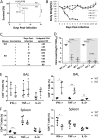
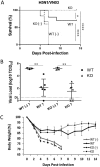
Anti-galactose-α-1,3-galactose (anti-α-Gal) antibody is naturally expressed at a high level in humans. It constitutes about 1% of immunoglobulins found in human blood. Here, we designed a live attenuated influenza virus vaccine that can generate α-Gal epitopes in infected cells in order to facilitate opsonization of infected cells, thereby enhancing vaccine-induced immune responses. In the presence of normal human sera, cells infected with this mutant can enhance phagocytosis of human macrophages and cytotoxicity of NK cells in vitro Using a knockout mouse strain that allows expression of anti-α-Gal antibody in vivo, we showed that this strategy can increase vaccine immunogenicity and the breadth of protection. This vaccine can induce 100% protection against a lethal heterosubtypic group 1 (H5) or group 2 (mouse-adapted H3) influenza virus challenge in the mouse model. In contrast, its heterosubtypic protective effect in wild-type or knockout mice that do not have anti-α-Gal antibody expression is only partial, demonstrating that the enhanced vaccine-induced protection requires anti-α-Gal antibody upon vaccination. Anti-α-Gal-expressing knockout mice immunized with this vaccine produce robust humoral and cell-mediated responses upon a lethal virus challenge. This vaccine can stimulate CD11blo/- pulmonary dendritic cells, which are known to be crucial for clearance of influenza virus. Our approach provides a novel strategy for developing next-generation influenza virus vaccines.IMPORTANCE Influenza A viruses have multiple HA subtypes that are antigenically diverse. Classical influenza virus vaccines are subtype specific, and they cannot induce satisfactory heterosubtypic immunity against multiple influenza virus subtypes. Here, we developed a live attenuated H1N1 influenza virus vaccine that allows the expression of α-Gal epitopes by infected cells. Anti-α-Gal antibody is naturally produced by humans. In the presence of this antibody, human cells infected with this experimental vaccine virus can enhance several antibody-mediated immune responses in vitro Importantly, mice expressing anti-α-Gal antibody in vivo can be fully protected by this H1N1 vaccine against a lethal H5 or H3 virus challenge. Our work demonstrates a new strategy for using a single influenza virus strain to induce broadly cross-reactive immune responses against different influenza virus subtypes.
Keywords: immunology; influenza; influenza virus vaccines; live vector vaccines; universal vaccine.
Copyright © 2020 Yan et al.
Figures





References
-
- World Health Organization. 2009. Assessing the severity of an influenza pandemic. https://www.who.int/csr/disease/swineflu/assess/disease_swineflu_assess_....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
