Emergence of a Plasmid-Encoded Resistance-Nodulation-Division Efflux Pump Conferring Resistance to Multiple Drugs, Including Tigecycline, in Klebsiella pneumoniae
- PMID: 32127452
- PMCID: PMC7064769
- DOI: 10.1128/mBio.02930-19
Emergence of a Plasmid-Encoded Resistance-Nodulation-Division Efflux Pump Conferring Resistance to Multiple Drugs, Including Tigecycline, in Klebsiella pneumoniae
Abstract
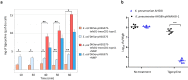
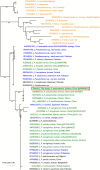
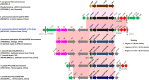
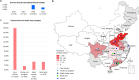
Transporters belonging to the chromosomally encoded resistance-nodulation-division (RND) superfamily mediate multidrug resistance in Gram-negative bacteria. However, the cotransfer of large gene clusters encoding RND-type pumps from the chromosome to a plasmid appears infrequent, and no plasmid-mediated RND efflux pump gene cluster has yet been found to confer resistance to tigecycline. Here, we identified a novel RND efflux pump gene cluster, designated tmexCD1-toprJ1, on plasmids from five pandrug-resistant Klebsiella pneumoniae isolates of animal origin. TMexCD1-TOprJ1 increased (by 4- to 32-fold) the MICs of tetracyclines (including tigecycline and eravacycline), quinolones, cephalosporins, and aminoglycosides for K.pneumoniae, Escherichia coli, and Salmonella TMexCD1-TOprJ1 is closely related (64.5% to 77.8% amino acid identity) to the MexCD-OprJ efflux pump encoded on the chromosome of Pseudomonas aeruginosa In an IncFIA plasmid, pHNAH8I, the tmexCD1-toprJ1 gene cluster lies adjacent to two genes encoding site-specific integrases, which may have been responsible for its acquisition. Expression of TMexCD1-TOprJ1 in E. coli resulted in increased tigecycline efflux and in K. pneumoniae negated the efficacy of tigecycline in an in vivo infection model. Expression of TMexCD1-TOprJ1 reduced the growth of E. coli and Salmonella but not K. pneumoniaetmexCD1-toprJ1-positive Enterobacteriaceae isolates were rare in humans (0.08%) but more common in chicken fecal (14.3%) and retail meat (3.4%) samples. Plasmid-borne tmexCD1-toprJ1-like gene clusters were identified in sequences in GenBank from Enterobacteriaceae and Pseudomonas strains from multiple continents. The possibility of further global dissemination of the tmexCD1-toprJ1 gene cluster and its analogues in Enterobacteriaceae via plasmids may be an important consideration for public health planning.IMPORTANCE In an era of increasing concerns about antimicrobial resistance, tigecycline is likely to have a critically important role in the treatment of carbapenem-resistant Enterobacteriaceae, the most problematic pathogens in human clinical settings-especially carbapenem-resistant K.pneumoniae Here, we identified a new plasmid-borne RND-type tigecycline resistance determinant, TMexCD1-TOprJ1, which is widespread among K. pneumoniae isolates from food animals. tmexCD1-toprJ1 appears to have originated from the chromosome of a Pseudomonas species and may have been transferred onto plasmids by adjacent site-specific integrases. Although tmexCD1-toprJ1 still appears to be rare in human clinical isolates, considering the transferability of the tmexCD1-toprJ1 gene cluster and the broad substrate spectrum of TMexCD1-TOprJ1, further dissemination of this mobile tigecycline resistance determinant is possible. Therefore, from a "One Health" perspective, measures are urgently needed to monitor and control its further spread. The current low prevalence in human clinical isolates provides a precious time window to design and implement measures to tackle this.
Keywords: Enterobacteriaceae; antimicrobial agents; efflux pumps; mechanisms of resistance; multidrug resistance; plasmid-mediated resistance.
Copyright © 2020 Lv et al.
Figures


References
-
- Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, WHO Pathogens Priority List Working Group. 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. doi:10.1016/S1473-3099(17)30753-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

