Intermittent Lactobacilli-containing Vaginal Probiotic or Metronidazole Use to Prevent Bacterial Vaginosis Recurrence: A Pilot Study Incorporating Microscopy and Sequencing
- PMID: 32127550
- PMCID: PMC7054572
- DOI: 10.1038/s41598-020-60671-6
Intermittent Lactobacilli-containing Vaginal Probiotic or Metronidazole Use to Prevent Bacterial Vaginosis Recurrence: A Pilot Study Incorporating Microscopy and Sequencing
Abstract
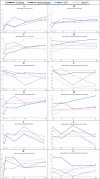
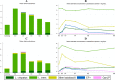
Bacterial vaginosis (BV) is associated with HIV acquisition and adverse pregnancy outcomes. Recurrence after metronidazole treatment is high. HIV-negative, non-pregnant Rwandan BV patients were randomized to four groups (n = 17/group) after seven-day oral metronidazole treatment: behavioral counseling only (control), or counseling plus intermittent use of oral metronidazole, Ecologic Femi+ vaginal capsule (containing multiple Lactobacillus and one Bifidobacterium species), or Gynophilus LP vaginal tablet (L. rhamnosus 35) for two months. Vaginal microbiota assessments at all visits included Gram stain Nugent scoring and 16S rRNA gene qPCR and HiSeq sequencing. All interventions were safe. BV (Nugent 7-10) incidence was 10.18 per person-year at risk in the control group, and lower in the metronidazole (1.41/person-year; p = 0.004), Ecologic Femi+ (3.58/person-year; p = 0.043), and Gynophilus LP groups (5.36/person-year; p = 0.220). In mixed effects models adjusted for hormonal contraception/pregnancy, sexual risk-taking, and age, metronidazole and Ecologic Femi+ users, each compared to controls, had higher Lactobacillus and lower BV-anaerobes estimated concentrations and/or relative abundances, and were less likely to have a dysbiotic vaginal microbiota type by sequencing. Inter-individual variability was high and effects disappeared soon after intervention cessation. Lactobacilli-based vaginal probiotics warrant further evaluation because, in contrast to antibiotics, they are not expected to negatively affect gut microbiota or cause antimicrobial resistance.
Conflict of interest statement
AN is employed by Biose (owner of trial product GynLP) and EL by Winclove Probiotics BV (owner of trial product EF+). AN and JR have financial and/or intellectual investments in competing products. The other authors report no competing interests.
Figures

References
-
- World Health Organization. Guidelines for the management of sexually transmitted infections, http://applications.emro.who.int/aiecf/web79.pdf (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

