Visuocortical tuning to a threat-related feature persists after extinction and consolidation of conditioned fear
- PMID: 32127551
- PMCID: PMC7054355
- DOI: 10.1038/s41598-020-60597-z
Visuocortical tuning to a threat-related feature persists after extinction and consolidation of conditioned fear
Abstract
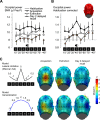
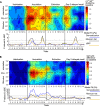
Neurons in the visual cortex sharpen their orientation tuning as humans learn aversive contingencies. A stimulus orientation (CS+) that reliably predicts an aversive noise (unconditioned stimulus: US) is selectively enhanced in lower-tier visual cortex, while similar unpaired orientations (CS-) are inhibited. Here, we examine in male volunteers how sharpened visual processing is affected by fear extinction learning (where no US is presented), and how fear and extinction memory undergo consolidation one day after the original learning episode. Using steady-state visually evoked potentials from electroencephalography in a fear generalization task, we found that extinction learning prompted rapid changes in orientation tuning: Both conditioned visuocortical and skin conductance responses to the CS+ were strongly reduced. Next-day re-testing (delayed recall) revealed a brief but precise return-of-tuning to the CS+ in visual cortex accompanied by a brief, more generalized return-of-fear in skin conductance. Explorative analyses also showed persistent tuning to the threat cue in higher visual areas, 24 h after successful extinction, outlasting peripheral responding. Together, experience-based changes in the sensitivity of visual neurons show response patterns consistent with memory consolidation and spontaneous recovery, the hallmarks of long-term neural plasticity.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Learning pain-related fear: neural mechanisms mediating rapid differential conditioning, extinction and reinstatement processes in human visceral pain.Neurobiol Learn Mem. 2014 Dec;116:36-45. doi: 10.1016/j.nlm.2014.08.003. Epub 2014 Aug 13. Neurobiol Learn Mem. 2014. PMID: 25128878
-
Human Sensory Cortex Contributes to the Long-Term Storage of Aversive Conditioning.J Neurosci. 2021 Apr 7;41(14):3222-3233. doi: 10.1523/JNEUROSCI.2325-20.2021. Epub 2021 Feb 23. J Neurosci. 2021. PMID: 33622774 Free PMC article.
-
Enhancing extinction learning: Occasional presentations of the unconditioned stimulus during extinction eliminate spontaneous recovery, but not necessarily reacquisition of fear.Behav Res Ther. 2018 Sep;108:29-39. doi: 10.1016/j.brat.2018.07.001. Epub 2018 Jul 3. Behav Res Ther. 2018. PMID: 29981936
-
Biological preparedness and resistance to extinction of skin conductance responses conditioned to fear relevant animal pictures: A systematic review.Neurosci Biobehav Rev. 2018 Dec;95:430-437. doi: 10.1016/j.neubiorev.2018.10.017. Epub 2018 Oct 28. Neurosci Biobehav Rev. 2018. PMID: 30381252
-
Fear conditioning and extinction: emotional states encoded by distinct signaling pathways.Trends Neurosci. 2012 Mar;35(3):145-55. doi: 10.1016/j.tins.2011.10.003. Epub 2011 Nov 25. Trends Neurosci. 2012. PMID: 22118930 Free PMC article. Review.
Cited by
-
Fear conditioning prompts sparser representations of conditioned threat in primary visual cortex.Soc Cogn Affect Neurosci. 2020 Nov 6;15(9):950-964. doi: 10.1093/scan/nsaa122. Soc Cogn Affect Neurosci. 2020. PMID: 32901822 Free PMC article.
-
Quantifying Population-level Neural Tuning Functions Using Ricker Wavelets and the Bayesian Bootstrap.bioRxiv [Preprint]. 2024 May 23:2024.05.22.595429. doi: 10.1101/2024.05.22.595429. bioRxiv. 2024. Update in: J Neurosci Methods. 2025 Jan;413:110303. doi: 10.1016/j.jneumeth.2024.110303. PMID: 38826264 Free PMC article. Updated. Preprint.
-
Quantifying population-level neural tuning functions using Ricker wavelets and the Bayesian bootstrap.J Neurosci Methods. 2025 Jan;413:110303. doi: 10.1016/j.jneumeth.2024.110303. Epub 2024 Oct 19. J Neurosci Methods. 2025. PMID: 39428077 Free PMC article.
-
Neurophysiological and Autonomic Dynamics of Threat Processing during Sustained Social Fear Generalization.J Cogn Neurosci. 2025 Feb 1;37(2):482-497. doi: 10.1162/jocn_a_02276. J Cogn Neurosci. 2025. PMID: 39536160
-
Effects of phase synchronization and frequency specificity in the encoding of conditioned fear-a web-based fear conditioning study.PLoS One. 2023 Mar 3;18(3):e0281644. doi: 10.1371/journal.pone.0281644. eCollection 2023. PLoS One. 2023. PMID: 36867619 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

