Modified mRNA/lipid nanoparticle-based vaccines expressing respiratory syncytial virus F protein variants are immunogenic and protective in rodent models of RSV infection
- PMID: 32128257
- PMCID: PMC7021756
- DOI: 10.1038/s41541-020-0163-z
Modified mRNA/lipid nanoparticle-based vaccines expressing respiratory syncytial virus F protein variants are immunogenic and protective in rodent models of RSV infection
Abstract
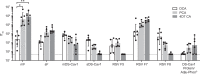
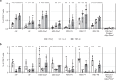
The RSV Fusion (F) protein is a target for neutralizing antibody responses and is a focus for vaccine discovery; however, the process of RSV entry requires F to adopt a metastable prefusion form and transition to a more stable postfusion form, which displays less potent neutralizing epitopes. mRNA vaccines encode antigens that are translated by host cells following vaccination, which may allow conformational transitions similar to those observed during natural infection to occur. Here we evaluate a panel of chemically modified mRNA vaccines expressing different forms of the RSV F protein, including secreted, membrane associated, prefusion-stabilized, and non-stabilized structures, for conformation, immunogenicity, protection, and safety in rodent models. Vaccination with mRNA encoding native RSV F elicited antibody responses to both prefusion- and postfusion-specific epitopes, suggesting that this antigen may adopt both conformations in vivo. Incorporating prefusion stabilizing mutations further shifts the immune response toward prefusion-specific epitopes, but does not impact neutralizing antibody titer. mRNA vaccine candidates expressing either prefusion stabilized or native forms of RSV F protein elicit robust neutralizing antibody responses in both mice and cotton rats, similar to levels observed with a comparable dose of adjuvanted prefusion stabilized RSV F protein. In contrast to the protein subunit vaccine, mRNA-based vaccines elicited robust CD4+ and CD8+ T-cell responses in mice, highlighting a potential advantage of the technology for vaccines requiring a cellular immune response for efficacy.
Keywords: RNA vaccines; Vaccines; Virology.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsA.S.E., P.J.C., M.P.C., D.W., D.J.D., C.C., G.O., J.G., R.S., S.T., Z.W., J.A., L.Z., J.A.F., K.S.C., D.C.F., K.V., A.H.L., J.S.S., M.E.G., D.H., A.J.B. are all employees and/or stockholders of Merck & Co., Inc., Kenilworth, NJ, USA. C.A.S., K.B., and G.C. are employees and/or stockholders of Moderna Inc., Cambridge, MA, USA. G.C., K.B., A.S.E., D.W. and A.J.B. are co-inventors on a patent application related to this work.40
Figures



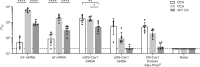

Similar articles
-
Flexible RSV Prefusogenic Fusion Glycoprotein Exposes Multiple Neutralizing Epitopes that May Collectively Contribute to Protective Immunity.Vaccines (Basel). 2020 Oct 14;8(4):607. doi: 10.3390/vaccines8040607. Vaccines (Basel). 2020. PMID: 33066540 Free PMC article.
-
A monomeric uncleaved respiratory syncytial virus F antigen retains prefusion-specific neutralizing epitopes.J Virol. 2014 Oct;88(20):11802-10. doi: 10.1128/JVI.01225-14. Epub 2014 Jul 30. J Virol. 2014. PMID: 25078705 Free PMC article.
-
Alternative Virus-Like Particle-Associated Prefusion F Proteins as Maternal Vaccines for Respiratory Syncytial Virus.J Virol. 2019 Nov 13;93(23):e00914-19. doi: 10.1128/JVI.00914-19. Print 2019 Dec 1. J Virol. 2019. PMID: 31511382 Free PMC article.
-
The third pandemic: The respiratory syncytial virus landscape and specific considerations for the allergist/immunologist.Allergy Asthma Proc. 2023 Jul 26;44(4):220-228. doi: 10.2500/aap.2023.44.230030. Allergy Asthma Proc. 2023. PMID: 37236777 Review.
-
Neutralizing epitopes on the respiratory syncytial virus fusion glycoprotein.Curr Opin Virol. 2015 Apr;11:70-5. doi: 10.1016/j.coviro.2015.03.002. Epub 2015 Mar 26. Curr Opin Virol. 2015. PMID: 25819327 Free PMC article. Review.
Cited by
-
Evaluation of adenoviral vector Ad19a encoding RSV-F as novel vaccine against respiratory syncytial virus.NPJ Vaccines. 2024 Oct 29;9(1):205. doi: 10.1038/s41541-024-01001-z. NPJ Vaccines. 2024. PMID: 39472590 Free PMC article.
-
Nano-Enabled COVID-19 Vaccines: Meeting the Challenges of Durable Antibody Plus Cellular Immunity and Immune Escape.ACS Nano. 2021 Apr 27;15(4):5793-5818. doi: 10.1021/acsnano.1c01845. Epub 2021 Apr 1. ACS Nano. 2021. PMID: 33793189 Free PMC article.
-
AntigenBoost: enhanced mRNA-based antigen expression through rational amino acid substitution.Brief Bioinform. 2024 Sep 23;25(6):bbae468. doi: 10.1093/bib/bbae468. Brief Bioinform. 2024. PMID: 39400114 Free PMC article.
-
An Optimized FI-RSV Vaccine Effectively Protects Cotton Rats and BALB/c Mice without Causing Enhanced Respiratory Disease.Viruses. 2022 Sep 20;14(10):2085. doi: 10.3390/v14102085. Viruses. 2022. PMID: 36298640 Free PMC article.
-
Prefusion F-Based Polyanhydride Nanovaccine Induces Both Humoral and Cell-Mediated Immunity Resulting in Long-Lasting Protection against Respiratory Syncytial Virus.J Immunol. 2021 May 1;206(9):2122-2134. doi: 10.4049/jimmunol.2100018. Epub 2021 Apr 7. J Immunol. 2021. PMID: 33827894 Free PMC article.
References
-
- Mazur NI, et al. Respiratory Syncytial Virus Network (ReSViNET) Foundation. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect. Dis. 2018;18:e295–e311. - PubMed
-
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005;352:1749–1759. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

