The hidden costs of dietary restriction: Implications for its evolutionary and mechanistic origins
- PMID: 32128403
- PMCID: PMC7034997
- DOI: 10.1126/sciadv.aay3047
The hidden costs of dietary restriction: Implications for its evolutionary and mechanistic origins
Abstract
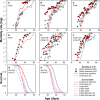
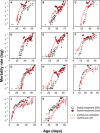
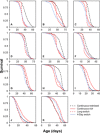
Dietary restriction (DR) extends life span across taxa. Despite considerable research, universal mechanisms of DR have not been identified, limiting its translational potential. Guided by the conviction that DR evolved as an adaptive, pro-longevity physiological response to food scarcity, biomedical science has interpreted DR as an activator of pro-longevity molecular pathways. Current evolutionary theory predicts that organisms invest in their soma during DR, and thus when resource availability improves, should outcompete rich-fed controls in survival and/or reproduction. Testing this prediction in Drosophila melanogaster (N > 66,000 across 11 genotypes), our experiments revealed substantial, unexpected mortality costs when flies returned to a rich diet following DR. The physiological effects of DR should therefore not be interpreted as intrinsically pro-longevity, acting via somatic maintenance. We suggest DR could alternatively be considered an escape from costs incurred under nutrient-rich conditions, in addition to costs associated with DR.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution License 4.0 (CC BY).
Figures

References
-
- Nakagawa S., Lagisz M., Hector K. L., Spencer H. G., Comparative and meta-analytic insights into life extension via dietary restriction. Aging Cell 11, 401–409 (2012). - PubMed
-
- Solon-Biet S. M., McMahon A. C., Ballard J. W. O., Ruohonen K., Wu L. E., Cogger V. C., Warren A., Huang X., Pichaud N., Melvin R. G., Gokarn R., Khalil M., Turner N., Cooney G. J., Sinclair D. A., Raubenheimer D., Le Couteur D. G., Simpson S. J., The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 19, 418–430 (2014). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

