Enhanced CRISPR-Cas9 correction of Duchenne muscular dystrophy in mice by a self-complementary AAV delivery system
- PMID: 32128412
- PMCID: PMC7030925
- DOI: 10.1126/sciadv.aay6812
Enhanced CRISPR-Cas9 correction of Duchenne muscular dystrophy in mice by a self-complementary AAV delivery system
Abstract
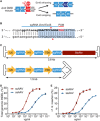
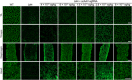
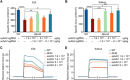
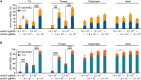
Duchenne muscular dystrophy (DMD) is a lethal neuromuscular disease caused by mutations in the dystrophin gene (DMD). Previously, we applied CRISPR-Cas9-mediated "single-cut" genome editing to correct diverse genetic mutations in animal models of DMD. However, high doses of adeno-associated virus (AAV) are required for efficient in vivo genome editing, posing challenges for clinical application. In this study, we packaged Cas9 nuclease in single-stranded AAV (ssAAV) and CRISPR single guide RNAs in self-complementary AAV (scAAV) and delivered this dual AAV system into a mouse model of DMD. The dose of scAAV required for efficient genome editing were at least 20-fold lower than with ssAAV. Mice receiving systemic treatment showed restoration of dystrophin expression and improved muscle contractility. These findings show that the efficiency of CRISPR-Cas9-mediated genome editing can be substantially improved by using the scAAV system. This represents an important advancement toward therapeutic translation of genome editing for DMD.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures

References
-
- Hoffman E. P., Brown R. H. Jr., Kunkel L. M., Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell 51, 919–928 (1987). - PubMed
-
- Koenig M., Hoffman E. P., Bertelson C. J., Monaco A. P., Feener C., Kunkel L. M., Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 50, 509–517 (1987). - PubMed
-
- Guiraud S., Aartsma-Rus A., Vieira N. M., Davies K. E., van Ommen G.-J. B., Kunkel L. M., The pathogenesis and therapy of muscular dystrophies. Annu. Rev. Genomics Hum. Genet. 16, 281–308 (2015). - PubMed
-
- Campbell K. P., Kahl S. D., Association of dystrophin and an integral membrane glycoprotein. Nature 338, 259–262 (1989). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

