Egocentric boundary vector tuning of the retrosplenial cortex
- PMID: 32128423
- PMCID: PMC7035004
- DOI: 10.1126/sciadv.aaz2322
Egocentric boundary vector tuning of the retrosplenial cortex
Abstract
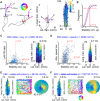
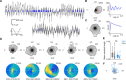
The retrosplenial cortex is reciprocally connected with multiple structures implicated in spatial cognition, and damage to the region itself produces numerous spatial impairments. Here, we sought to characterize spatial correlates of neurons within the region during free exploration in two-dimensional environments. We report that a large percentage of retrosplenial cortex neurons have spatial receptive fields that are active when environmental boundaries are positioned at a specific orientation and distance relative to the animal itself. We demonstrate that this vector-based location signal is encoded in egocentric coordinates, is localized to the dysgranular retrosplenial subregion, is independent of self-motion, and is context invariant. Further, we identify a subpopulation of neurons with this response property that are synchronized with the hippocampal theta oscillation. Accordingly, the current work identifies a robust egocentric spatial code in retrosplenial cortex that can facilitate spatial coordinate system transformations and support the anchoring, generation, and utilization of allocentric representations.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures




References
-
- Hafting T., Fyhn M., Molden S., Moser M.-B., Moser E. I., Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806 (2005). - PubMed
-
- O’Keefe J., Dostrovsky J., The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175 (1971). - PubMed
-
- Morris R. G. M., Garrud P., Rawlins J. N. P., O’Keefe J., Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683 (1982). - PubMed
-
- Steffenach H.-A., Witter M., Moser M.-B., Moser E. I., Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron 45, 301–313 (2005). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

