Indicators of retention in remote digital health studies: a cross-study evaluation of 100,000 participants
- PMID: 32128451
- PMCID: PMC7026051
- DOI: 10.1038/s41746-020-0224-8
Indicators of retention in remote digital health studies: a cross-study evaluation of 100,000 participants
Abstract
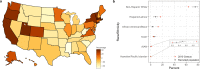
Digital technologies such as smartphones are transforming the way scientists conduct biomedical research. Several remotely conducted studies have recruited thousands of participants over a span of a few months allowing researchers to collect real-world data at scale and at a fraction of the cost of traditional research. Unfortunately, remote studies have been hampered by substantial participant attrition, calling into question the representativeness of the collected data including generalizability of outcomes. We report the findings regarding recruitment and retention from eight remote digital health studies conducted between 2014-2019 that provided individual-level study-app usage data from more than 100,000 participants completing nearly 3.5 million remote health evaluations over cumulative participation of 850,000 days. Median participant retention across eight studies varied widely from 2-26 days (median across all studies = 5.5 days). Survival analysis revealed several factors significantly associated with increase in participant retention time, including (i) referral by a clinician to the study (increase of 40 days in median retention time); (ii) compensation for participation (increase of 22 days, 1 study); (iii) having the clinical condition of interest in the study (increase of 7 days compared with controls); and (iv) older age (increase of 4 days). Additionally, four distinct patterns of daily app usage behavior were identified by unsupervised clustering, which were also associated with participant demographics. Most studies were not able to recruit a sample that was representative of the race/ethnicity or geographical diversity of the US. Together these findings can help inform recruitment and retention strategies to enable equitable participation of populations in future digital health research.
Keywords: Health care; Medical research.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

