Magnetic resonance (MR) safety and compatibility of a novel iron bioresorbable scaffold
- PMID: 32128465
- PMCID: PMC7044471
- DOI: 10.1016/j.bioactmat.2020.02.011
Magnetic resonance (MR) safety and compatibility of a novel iron bioresorbable scaffold
Abstract
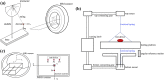
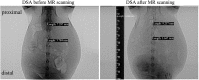
Fully bioresorbable scaffolds have been designed to overcome the limitations of traditional drug-eluting stents (DESs), which permanently cage the native vessel wall and pose possible complications. The ultrathin-strut designed sirolimus-eluting iron bioresorbable coronary scaffold system (IBS) shows comparable mechanical properties to traditional DESs and exhibits an adaptive degradation profile during target vessel healing, which makes it a promising candidate in all-comers patient population. For implanted medical devices, magnetic resonance (MR) imaging properties, including MR safety and compatibility, should be evaluated before its clinical use, especially for devices with intrinsic ferromagnetism. In this study, MR safety and compatibility of the IBS scaffold were evaluated based on a series of well-designed in-vitro, ex-vivo and in-vivo experiments, considering possible risks, including scaffold movement, over-heating, image artifact, and possible vessel injury, under typical MR condition. Traditional ASTM standards for MR safety and compatibility evaluation of intravascular devices were referred, but not only limited to that. The unique time-relevant MR properties of bioresorbable scaffolds were also discussed. Possible forces imposed on the scaffold during MR scanning and MR image artifacts gradually decreased along with scaffold degradation/absorption. Rigorous experiments designed based on a scientifically based rationale revealed that the IBS scaffold is MR conditional, though not MR compatible before complete absorption. The methodology used in the present study can give insight into the MR evaluation of magnetic scaffolds (bioresorbable) or stents (permanent).
Keywords: Artifact; Iron bioresorbable scaffold; MR compatibility; MR safety; Magnetic resonance imaging (MRI).
© 2020 Production and hosting by Elsevier B.V. on behalf of KeAi Communications Co., Ltd.
Conflict of interest statement
The authors declared no conflict of interest.
Figures









References
-
- Garg S., Serruys P.W. Coronary stents: looking forward. J. Am. Coll. Cardiol. 2010;56(10):S43–S78. - PubMed
-
- Zheng Y.F., Gu X.N., Witte F. Biodegradable metals. Math. Sci. Eng. R. 2014;77:1–34. 0.
-
- Indolfi C., Rosa S.D., Colombo A. Bioresorbable vascular scaffolds - basic concepts and clinical outcome. Nat. Rev. Cardiol. 2016;13(12):719–729. - PubMed
-
- Serruys P.W.J.C., Onuma Y. CRC Press; 2017. Bioresorbable Scaffolds: from Basic Concept to Clinical Applications.
-
- Zhu Y.Q., Yang K., Cheng R.Y., Xiang Y., Yuan T.W., Cheng Y.S., Sarmento B., Cui W.G. The current status of biodegradable stent to treat benign luminal disease. Mater. Today. 2017;20(9):516–529.
LinkOut - more resources
Full Text Sources

