Probiotics for maintenance of remission in ulcerative colitis
- PMID: 32128794
- PMCID: PMC7059960
- DOI: 10.1002/14651858.CD007443.pub3
Probiotics for maintenance of remission in ulcerative colitis
Abstract
Background: Ulcerative colitis is an inflammatory condition affecting the colon, with an annual incidence of approximately 10 to 20 per 100,000 people. The majority of people with ulcerative colitis can be put into remission, leaving a group who do not respond to first- or second-line therapies. There is a significant proportion of people who experience adverse effects with current therapies. Consequently, new alternatives for the treatment of ulcerative colitis are constantly being sought. Probiotics are live microbial feed supplements that may beneficially affect the host by improving intestinal microbial balance, enhancing gut barrier function and improving local immune response.
Objectives: The primary objective was to determine the efficacy of probiotics compared to placebo, no treatment, or any other intervention for the maintenance of remission in people with ulcerative colitis. The secondary objective was to assess the occurrence of adverse events associated with the use of probiotics.
Search methods: We searched CENTRAL, MEDLINE, Embase, and two other databases on 31 October 2019. We contacted authors of relevant studies and manufacturers of probiotics regarding ongoing or unpublished trials that may be relevant to the review, and we searched ClinicalTrials.gov. We also searched references of trials for any additional trials.
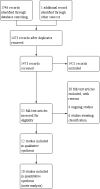
Selection criteria: Randomised controlled trials (RCTs) that compared probiotics against placebo or any other intervention, in both adults and children, for the maintenance of remission in ulcerative colitis were eligible for inclusion. Maintenance therapy had to be for a minimum of three months when remission has been established by any clinical, endoscopic,histological or radiological relapse as defined by study authors.
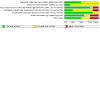
Data collection and analysis: Two review authors independently conducted data extraction and 'Risk of bias' assessment of included studies. We analysed data using Review Manager 5. We expressed dichotomous and continuous outcomes as risk ratios (RRs) and mean differences (MDs) with 95% confidence intervals (CIs). We assessed the certainty of the evidence using the GRADE methodology.
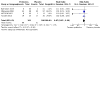
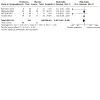
Main results: In this review, we included 12 studies (1473 randomised participants) that met the inclusion criteria. Participants were mostly adults. The studies compared probiotics to placebo, probiotics to 5-aminosalicylic acid (5-ASA) and a combination of probiotics and 5-ASA to 5-ASA. The studies ranged in length from 12 to 52 weeks. The average age of participants was between 32 and 51, with a range between 18 and 88 years. Seven studies investigated a single bacterial strain, and five studies considered mixed preparations of multiple strains. The risk of bias was high in all except three studies due to selective reporting, incomplete outcome data and lack of blinding. This resulted in low- to very low-certainty of evidence. It is uncertain if there is any difference in occurrence of clinical relapse when probiotics are compared with placebo (RR 0.87, 95% CI 0.63 to 1.18; 4 studies, 361 participants; very low-certainty evidence (downgraded for risk of bias, imbalance in baseline characteristics and imprecision)). It is also uncertain whether probiotics lead to a difference in the number of people who maintain clinical remission compared with placebo (RR 1.16, 95% CI 0.98 to 1.37; 2 studies, 141 participants; very low-certainty evidence (downgraded for risk of bias, imbalance in baseline characteristics and imprecision)). When probiotics are compared with 5-ASA, there may be little or no difference in clinical relapse (RR 1.01, 95% CI 0.84 to 1.22; 2 studies, 452 participants; low-certainty evidence) and maintenance of clinical remission (RR 1.06, 95% CI 0.90 to 1.25; 1 study, 125 participants; low-certainty evidence). It is uncertain if there is any difference in clinical relapse when probiotics, combined with 5-ASA are compared with 5-ASA alone (RR 1.11, 95% CI 0.66 to 1.87; 2 studies, 242 participants; very low-certainty evidence (downgraded due to risk of bias and imprecision)). There may be little or no difference in maintenance of remission when probiotics, combined with 5-ASA, are compared with 5-ASA alone (RR 1.05, 95% CI 0.89 to 1.24; 1 study, 122 participants; low-certainty evidence). Where reported, most of the studies which compared probiotics with placebo recorded no serious adverse events or withdrawals due to adverse events. For the comparison of probiotics and 5-ASA, one trial reported 11/110 withdrawals due to adverse events with probiotics and 11/112 with 5-ASA (RR 1.02, 95% CI 0.46 to 2.25; 222 participants; very low-certainty evidence). Discontinuation of therapy was due to gastrointestinal symptoms. One study (24 participants) comparing probiotics combined with 5-ASA with 5-ASA alone, reported no withdrawals due to adverse events; and two studies reported two withdrawals in the probiotic arm, due to avascular necrosis of bilateral femoral head and pulmonary thromboembolism (RR 5.29, 95% CI 0.26 to 107.63; 127 participants; very low-certainty evidence). Health-related quality of life and need for additional therapy were reported infrequently.
Authors' conclusions: The effectiveness of probiotics for the maintenance of remission in ulcerative colitis remains unclear. This is due to low- to very low-certainty evidence from poorly conducted studies, which contribute limited amounts of data from a small number of participants. Future trials comparing probiotics with 5-ASA rather than placebo will better reflect conventional care given to people with ulcerative colitis. Appropriately powered studies with a minimum length of 12 months are needed.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Zipporah Iheozor‐Ejiofor: my employment at the University of Central Lancashire is funded by the National Institute for Health Research (NIHR) UK and focuses on high priority Cochrane Reviews in Inflammatory Bowel Disease.
Lakhbir Kaur: none known
Morris Gordon: received travel grants from various companies to attend scientific meetings in the last three years, including Biogaia, Synergy, Tillots, Ferring and Allergan. None of these companies have had any involvement in the planning, completion, analysis or write up of this or any other reviews. This review has been completed as part of a UK funded National Institute for Health Research (NIHR) Cochrane Programme grant, with some time funded.
Patricia Baines: none known
Vasiliki Sinopoulou: none known
Anthony Akobeng: none known
Figures













Update of
-
Probiotics for maintenance of remission in ulcerative colitis.Cochrane Database Syst Rev. 2011 Dec 7;(12):CD007443. doi: 10.1002/14651858.CD007443.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2020 Mar 4;3:CD007443. doi: 10.1002/14651858.CD007443.pub3. PMID: 22161412 Updated.
Similar articles
-
Probiotics for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2020 Mar 4;3(3):CD005573. doi: 10.1002/14651858.CD005573.pub3. Cochrane Database Syst Rev. 2020. PMID: 32128795 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis.Cochrane Database Syst Rev. 2020 Aug 28;8(8):CD000544. doi: 10.1002/14651858.CD000544.pub5. Cochrane Database Syst Rev. 2020. PMID: 32856298 Free PMC article.
-
Oral 5-aminosalicylic acid for maintenance of surgically-induced remission in Crohn's disease.Cochrane Database Syst Rev. 2019 Jun 20;6(6):CD008414. doi: 10.1002/14651858.CD008414.pub3. Cochrane Database Syst Rev. 2019. PMID: 31220875 Free PMC article.
-
Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2020 Aug 12;8(8):CD000543. doi: 10.1002/14651858.CD000543.pub5. Cochrane Database Syst Rev. 2020. PMID: 32786164 Free PMC article.
Cited by
-
Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body.Front Immunol. 2021 Feb 26;12:578386. doi: 10.3389/fimmu.2021.578386. eCollection 2021. Front Immunol. 2021. PMID: 33717063 Free PMC article. Review.
-
Efficacy and Safety of Probiotics Combined With Traditional Chinese Medicine for Ulcerative Colitis: A Systematic Review and Meta-Analysis.Front Pharmacol. 2022 Mar 7;13:844961. doi: 10.3389/fphar.2022.844961. eCollection 2022. Front Pharmacol. 2022. PMID: 35321324 Free PMC article. Review.
-
Interactions between the Gut Microbiome, Lung Conditions, and Coronary Heart Disease and How Probiotics Affect These.Int J Mol Sci. 2021 Sep 8;22(18):9700. doi: 10.3390/ijms22189700. Int J Mol Sci. 2021. PMID: 34575864 Free PMC article. Review.
-
Probiotics in Pediatrics. A Review and Practical Guide.Nutrients. 2021 Jun 24;13(7):2176. doi: 10.3390/nu13072176. Nutrients. 2021. PMID: 34202742 Free PMC article. Review.
-
Improvement of ulcerative colitis control by searching and restricting of inflammatory trigger factors in daily clinical practice.Intest Res. 2023 Jan;21(1):100-109. doi: 10.5217/ir.2021.00110. Epub 2022 Nov 14. Intest Res. 2023. PMID: 36366932 Free PMC article.
References
References to studies included in this review
Bjarnason 2019 {published data only}
Copaci 2014 {published data only}
-
- Copaci I, Chiriac G. Maintenance of remission of ulcerative colitis: prebiotics and dietary fiber. United European Gastroenterology Journal 2014;1:A375.
Kruis 1997 {published data only}
-
- Kruis W, Schutz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Alimentary Pharmacology and Therapeutics 1997;11(5):853-8. - PubMed
Kruis 2004 {published data only}
Matsuoka 2018 {published data only}
NCT02361957 {unpublished data only}
-
- NCT02361957. The Effect of the Multispecies Probiotic Ecologic 825 Versus Placebo in Ulcerative Colitis Patients (CUPIDO). clinicaltrials.gov/ct2/show/NCT02361957 first posted 12 February 2015.
Shanahan 2006 {published data only}
-
- Shanahan F, Guarner F, Von Wright A, Vilpponen-Salmela T, O'Donoghue, D, Kiely B, et al. A one year, randomised, double-blind, placebo controlled trial of a Lactobacillus or a Bidifobacterium probiotic for maintenance of steroid-induced remission of ulcerative colitis. Gastroenterology 2006;130(4 Suppl 2):A44.
Vejdani 2017 {published data only}
-
- Vejdani R, Bahari A, Zadeh AM, Azmi M, Ebrahimi-Daryani N, Hashtroudi AA, et al. Effects of lactobacillus casei probiotic on mild to moderate ulcerative colitis: A placebo controlled study. Indian Journal of Medical Sciences January-March 2017;69(1):24-8.
Wildt 2011 {published data only}
-
- Wildt S, Nordgaard I, Hansen U, Brockmann E, Rumessen JJ. A randomised double-blind placebo-controlled trial with Lactobacillus acidophilus La-5 and Bifidobacterium animalis subsp. lactis BB-12 for maintenance of remission in ulcerative colitis. Journal of Crohn's and Colitis 2011;5(2):115-21. - PubMed
Yasushi 2015 {published data only}
Zocco 2003 {published data only}
-
- Zocco MA, dal Verme LZ, Armuzzi A, Nista EC, Papa A, Candelli M. Comparison of Lactobacillus GG and mesalazine in maintaining remission of ulcerative colitis and Crohn's disease. Gastroenterology 2003;124(4):A201.
Zocco 2006 {published data only}
-
- Zocco MA, dal Verme LZ, Cremonini F, Piscaglia AC, Nista EC, Candelli M, et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Alimentary Pharmacology and Therapeutics 2006;23(11):1567-74. - PubMed
References to studies excluded from this review
Ahmed 2013 {published data only}
-
- Ahmed J, Reddy BS, Molbak L, Leser TD, Macfie J. Impact of probiotic on colonic microflora in patients with colitis: A prospective double blind randomised crossover study. International Journal of Surgery 2013;11(10):1131-6. - PubMed
Ballini 2019 {published data only}
-
- Ballini A, Santacroce L, Cantore S, Bottalico L, Dipalma G, Tope S, et al. Probiotics efficacy on oxidative stress values in inflammatory bowel disease: a randomized double-blinded placebo-controlled pilot study. Endocrine, Metabolic & Immune Disorders - Drug Targets 2019;19(3):1-8. - PubMed
Bamba 2002 {published data only}
-
- Bamba T, Kanauchi O, Andoh A, Fujiyama Y. A new prebiotic from germinated barley for nutraceutical treatment of ulcerative colitis. Journal of Gastroenterology and Hepatology 2002;17(8):818-24. - PubMed
Cui 2004 {published data only}
Do 2010 {published data only}
-
- Do VT, Baird BG, Kockler DR. Probiotics for maintaining remission of ulcerative colitis in adults. Annals of Pharmacotherapy 2010;44(3):565-71. - PubMed
Faubion 2000 {published data only}
-
- Faubion WA, Sandborn WJ. Probiotic therapy with E. Coli for ulcerative colitis: take the good with the bad. Gastroenterology 2000;118(3):630-1. - PubMed
Folwaczny 2000 {published data only}
-
- Folwaczny C. Probiotics for prevention of ulcerative colitis recurrence: alternative medicine added to standard treatment. Zeitschfrift de Gastroenterologie 2000;38(6):547-50. - PubMed
Fujimori 2009 {published data only}
-
- Fujimori S, Gudis K, Mitsui K, Seo T, Yonezawa M, Tanaka S, et al. Randomized controlled trial on the efficacy of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis. Nutrition 2009;25(5):520-5. - PubMed
Henker 2008 {published data only}
-
- Henker J, Müller S, Laass MW, Schreiner A, Schulze J. Probiotic Escherichia coli Nissle 1917 (EcN) for successful remission maintenance of ulcerative colitis in children and adolescents: an open-label pilot study. Zeitschrift fur Gastroenterologie 2008;46(9):874-5. - PubMed
IRCT20120415009475N5 {published data only}
-
- IRCT20120415009475N5. Evaluation of the effects of Saccharomyces Boulardii on pain and quality of life in children with Inflammatory Bowel Disease (IBD) [Evaluation of the effects of lyophilized Saccharomyces Boulardii capsules on quality of life and clinical outcomes in children with inflammatory bowel disease (IBD)]. www.irct.ir/trial/32179 (registration date 10 October 2018). [IRCT: https://www.irct.ir/trial/32179]
Ishikawa 2002 {published data only}
-
- Ishikawa H, Akedo I, Umesaki Y, Tanaka R, Imaoka A, Otani T. Randomized controlled trial of the effect of bifidobacteria-fermented milk on ulcerative colitis. Journal of the American College of Nutrition 2002;22(1):56-63. - PubMed
Ishikawa 2011 {published data only}
-
- Ishikawa H, Matsumoto S, Ohashi Y, Imoaka A, Setoyama H, Umesaki Y, et al. Beneficial effects of probiotic bifidobacterium and galacto-oligosaccharide in patients with ulcerative colitis: A randomized controlled study. Digestion 2011;84(2):128-33. - PubMed
Li 2013 {published data only}
-
- Li K, Zhang CF, Xia YH, Li Z J, Han Y. Efficacy of probiotics on ulcerative colitis and its mechanism. Zhonghua Weichang Waike Zazhi 2013;16(4):336-9. - PubMed
Liu 2014 {published data only}
-
- Liu P, Sun L, Zhang ZH, Zhang P, Zhang J. Clinical efficacy of Salofalk combined with beneficial bacteria in patients with ulcerative colitis. World Chinese Journal of Digestology 2014;22(22):3344-8.
Miele 2009 {published data only}
-
- Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. American Journal of Gastroenterology 2009;104(2):437-43. - PubMed
NCT00268164 {published data only}
-
- NCT00268164. Lactobacilus acidophilus and Bifidobaceterium animalis subsp. Lactis maintenance treatment in ulcerative colitis. clinicaltrials.gov/show/NCT00268164 First received 22 December 2005.
NCT00374725 {published data only}
-
- NCT00374725. Treatment of Ulcerative Colitis With a Combination of Lactobacillus Rhamnosus and Lactobacillus Acidophilus. clinicaltrials.gov/show/NCT00374725 First posted 11 September 2006.
NCT00803829 {published data only}
-
- NCT00803829. Sunbiotic treatment of ulcerative colitis patients. clinialtrials.gov/show/NCT00803829 First posted 8 December 2008.
NCT00951548 {published data only}
-
- NCT00951548. Food Supplementation With VSL#3 as a Support to Standard Pharmaceutical Therapy in Ulcerative Colitis. clinicaltrials.gov/show/NCT00951548 First posted 4 August 2009.
NCT01772615 {published data only}
-
- NCT01772615. Treatment of ulcerative colitis with ciprofloxacin and E. Coli Nissle. clinicaltrials/gov/show/NCT01772615 First posted 21 january 2013.
Palumbo 2016 {published data only}
-
- Palumbo VD, Romeo M, Marino Gammazza A, Carini F, Damiani P, Damiano G et al. The long-term effects of probiotics in the therapy of ulcerative colitis: A clinical study. Biomedical Papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia 2016;160(3):372-7. - PubMed
Pelech 1998 {published data only}
-
- Pelech T, Fric P, Fixa B, Komarkova O. Comparison of mutaflor and mesalazine in the maintenance treatment of inactive ulcerative colitis. Prakticky Lekar 1998;78(10):556-8.
Rembacken 1999 {published data only}
-
- Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet 1999;354(9179):635-9. - PubMed
Rohatgi 2015 {published data only}
Sanchez‐Morales 2019 {published data only}
-
- Sanchez-Morales A, Perez-Ayala MF, Cruz-Martinez M, Arenas-Osuna J, Ramirez-Mendoza P, Ceniceros RA, et al. Efectividad de probioticos sobre sintomas, histologia, y tolerancia alimentaria en colitis ulcerativa. Revista Medica del Instituto Mexicano del Seguro Social 2019;57(1):9-14. - PubMed
Shadnoush 2013 {published data only}
Solovyeva 2014 {published data only}
-
- Solovyeva O. Probiotics can extend remission of ulcerative colitis. Journal of Crohn's and Colitis 2014;8:221.
Tursi 2010 {published data only}
-
- Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL3 as adjunctive to a standard pharmaceutical treatment: A double-blind, randomized, placebo-controlled study. American Journal of Gastroenterology 2010;105(10):2218-27. - PMC - PubMed
Venturi 1999 {published data only}
-
- Venturi A, Gionchetti P, Rizzello F, Johansson R, Zucconi E, Brigidi P, et al. Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Alimentary Pharmacology and Therapeutics 1999;13(8):1103-8. - PubMed
Zhang 2018a {published data only}
-
- Zhang J. Effects of Bifidobacterium quadruplex live bacteria tablets on Mayo score and hs-CRP, IL-4, IL-8 in patients with mild to moderate ulcerative colitis. World Chinese Journal of Digestology 2018;26(6):373-7.
References to studies awaiting assessment
Fan 2019 {published data only}
Fang 2018 {published data only}
-
- Fang W, Cai Q. Effect of mesalazine combined with bifid on inflammatory response and rectal-anal dynamics in patients with ulcerative colitis. World Chinese Journal of Digestology 2018;26(10):594-600.
Huang 2018 {published data only}
-
- Huang M, Chen Z, Lang C, Chen J, Yang B, Xue L, et al. Efficacy of mesalazine in combination with bifid triple viable capsules on ulcerative colitis and the resultant effect on the inflammatory factors. Pakistan Journal of Pharmaceutical Sciences 2018;31(6):2891-5. - PubMed
Shi 2018 {published data only}
-
- Shi XH, Tan FP, Jiang WH. Bacillus subtilis and Enterococcus faecium enteric-coated capsules combined with mesalazine for treatment of patients with ulcerative colitis: Efficacy and impact on serum levels of SOD, MDA, interleukins, and TNF-α. World Chinese Journal of Digestology 2018;26(12):748-54.
Yilmaz 2019 {published data only}
Zhang 2018b {published data only}
-
- Zhang Y, Wu M, Chen Y. Effects of adjuvant therapy with bifidobacterium quadruplex on lipid peroxidation injury indices, inflammatory factors and immune function in patients with ulcerative colitis. World Chinese Journal of Digestology 2018;26(22):1348-54.
References to ongoing studies
NCT03415711 {published data only}
-
- NCT03415711. PRObiotic VSL#3® for Maintenance of Clinical and Endoscopic REMission in Ulcerative Colitis (PROREM UC). clinicaltrials.gov/ct2/show/NCT03415711 First posted 30 January 2018.
NCT03565939 {published data only}
-
- NCT03565939. Probiotic Treatment of Ulcerative Colitis With Trichuris Suis Ova (TSO) (PROCTO). clinicaltrials.gov/ct2/show/NCT03565939 First posted 21 June 2018.
NCT03798210 {published data only}
-
- NCT03798210. Lactobacillus Reuteri ATCC PTA 4659 in Ulcerative Colitis (COLUS). clinicaltrials.gov/ct2/show/NCT03798210 First posted 9 January 2019.
NCT04006977 {published data only}
-
- Liang J. Multistrain Probiotics Reduces UC Depression and Anxiety Scores. clinicaltrials.gov/ct2/show/NCT04006977 First posted 5 July 2019.
Additional references
Baron 1964
Cleynen 2016
Cohen 2010
-
- Cohen RD. Systematic review: the costs of ulcerative colitis in Western countries. Alimentary Pharmaology and Therapeutics 2010;31(7):693-707. - PubMed
Dieleman 2003
Fabia 1993
-
- Fabia R, Ar'Rajab A, Johansson ML, Willen R, Andersson R, Molin G, et al. The effect of exogenous administration of Lactobacillus reuteri R2LC and oat fiber on acetic-induced colitis in the rat. Scandinavian Journal of Gastroenterology 1993;28(2):155-62. - PubMed
Fiorino 2016
GRADEpro GDT 2015 [Computer program]
-
- McMaster University GRADEpro GDT: GRADEpro Guideline Development Tool. McMaster University, 2015 (developed by Evidence Prime, Inc.).Available from gradepro.org.
Higgins 2003
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jairath 2017
-
- Jairath V, Zou GY, Parker CP, MacDonald JK, AlAmeel T, Al Beshir M et al. Placebo response and remission rates in randomised trials of induction and maintenance therapy for ulcerative colitis. Cochrane Database of Systematic Reviews 2017, Issue 9. [DOI: 10.1002/14651858.CD011572.pub2] - DOI - PMC - PubMed
Kennedy 2000
-
- Kennedy RJ, Hoper M, Deodhar K, Kirk SJ, Gardiner KR. Probiotic therapy fails to improve gut permeability in a hapten model of colitis. Scandinavian Journal of Gastroenterology 2000;35(12):1266-71. - PubMed
Loftus 2004
-
- Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004;126(6):1504-17. - PubMed
Madsen 1999
-
- Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology 1999;116(5):1107-14. - PubMed
Madsen 2001
-
- Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001;121(3):580-91. - PubMed
Mallon 2007
NICE 2019
-
- National Institute for Health and Care Excellence. Ulcerative Colitis Mangagement NG130. https://www.nice.org.uk/guidance/ng130 2019. - PubMed
Ong 2019
Panigrahi 2014
-
- Panigrahi P. Probiotics and prebiotics in neonatal necrotizing enterocolitis: new opportunities for translational research. Pathophysiology 2014;21(1):35-46. - PubMed
Pitkin 1999
-
- Pitkin RM, Branagan MA, Burmeister LF. Accuracy of data in abstracts of published research articles. JAMA 1999;281:1110-1. - PubMed
Review Manager 2014 [Computer program]
-
- The Cochrane Collaboration The Nordic Centre. Copenhagen: The Cochrane Collaboration, 13th June 2014.Available at https://community.cochrane.org/help/tools-and-software/revman-5.
Schultz 2002
-
- Schultz M, Veltkamp C, Dieleman LA, Grenther WB, Wyrick PB, Tonkonogy SL, et al. Lactobacillus plantarum 299V in the treatment and prevention of spontaneous colitis in interleukin-10-deficient mice. Inflammatory Bowel Diseases 2002;8(2):71-80. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shibolet 2002
-
- Shibolet O, Karmeli F, Eliakim R, Swennen E, Brigidi P, Gionchetti P, et al. Variable response to probiotics in two models of experimental colitis in rats. Inflammatory Bowel Diseases 2002;8(6):399-406. - PubMed
Truelove 1956
Turner 2018
-
- Turner D, Ruemmele FM, Orlanski-Meyer E, Griffiths AM, Carpi JM, Bronsky J, et al. Management of paediatric ulcerative colitis, Part 1: ambulatory care—an evidence-based guideline From European Crohn's and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2018;67(2):257-91. [DOI: 10.1097/mpg.0000000000002035] - DOI - PubMed
Ungaro 2016
References to other published versions of this review
Fagbemi 2008
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

