Probiotics for induction of remission in ulcerative colitis
- PMID: 32128795
- PMCID: PMC7059959
- DOI: 10.1002/14651858.CD005573.pub3
Probiotics for induction of remission in ulcerative colitis
Abstract
Background: Ulcerative colitis is an inflammatory condition affecting the colon, with an annual incidence of approximately 10 to 20 per 100,000 people. The majority of people with ulcerative colitis can be put into remission, leaving a group who do not respond to first- or second-line therapies. There is a significant proportion of people who experience adverse effects with current therapies. Consequently, new alternatives for the treatment of ulcerative colitis are constantly being sought. Probiotics are live microbial feed supplements that may beneficially affect the host by improving intestinal microbial balance, enhancing gut barrier function and improving local immune response.
Objectives: To assess the efficacy of probiotics compared with placebo or standard medical treatment (5-aminosalicylates, sulphasalazine or corticosteroids) for the induction of remission in people with active ulcerative colitis.
Search methods: We searched CENTRAL, MEDLINE, Embase, and two other databases on 31 October 2019. We contacted authors of relevant studies and manufacturers of probiotics regarding ongoing or unpublished trials that may be relevant to the review, and we searched ClinicalTrials.gov. We also searched references of trials for any additional trials.
Selection criteria: Randomised controlled trials (RCTs) investigating the effectiveness of probiotics compared to standard treatments or placebo in the induction of remission of active ulcerative colitis. We considered both adults and children, with studies reporting outcomes of clinical, endoscopic, histologic or surgical remission as defined by study authors DATA COLLECTION AND ANALYSIS: Two review authors independently conducted data extraction and 'Risk of bias' assessment of included studies. We analysed data using Review Manager 5. We expressed dichotomous and continuous outcomes as risk ratios (RRs) and mean differences (MDs) with 95% confidence intervals (CIs). We assessed the certainty of the evidence using the GRADE methodology.
Main results: In this review, we included 14 studies (865 randomised participants) that met the inclusion criteria. Twelve of the studies looked at adult participants and two studies looked at paediatric participants with mild to moderate ulcerative colitis, the average age was between 12.5 and 47.7 years. The studies compared probiotics to placebo, probiotics to 5-ASA and a combination of probiotics plus 5-ASA compared to 5-ASA alone. Seven studies used a single probiotic strain and seven used a mixture of strains. The studies ranged from two weeks to 52 weeks. The risk of bias was high for all except two studies due to allocation concealment, blinding of participants, incomplete reports of outcome data and selective reporting. This led to GRADE ratings of the evidence ranging from moderate to very low. Probiotics versus placebo Probiotics may induce clinical remission when compared to placebo (RR 1.73, 95% CI 1.19 to 2.54; 9 studies, 594 participants; low-certainty evidence; downgraded due to imprecision and risk of bias, number needed to treat for an additional beneficial outcome (NNTB) 5). Probiotics may lead to an improvement in clinical disease scores (RR 2.29, 95% CI 1.13 to 4.63; 2 studies, 54 participants; downgraded due to risk of bias and imprecision). There may be little or no difference in minor adverse events, but the evidence is of very low certainty (RR 1.04, 95% CI 0.42 to 2.59; 7 studies, 520 participants). Reported adverse events included abdominal bloating and discomfort. Probiotics did not lead to any serious adverse events in any of the seven studies that reported on it, however five adverse events were reported in the placebo arm of one study (RR 0.09, CI 0.01 to 1.66; 1 study, 526 participants; very low-certainty evidence; downgraded due to high risk of bias and imprecision). Probiotics may make little or no difference to withdrawals due to adverse events (RR 0.85, 95% CI 0.42 to 1.72; 4 studies, 401 participants; low-certainty evidence). Probiotics versus 5-ASA There may be little or no difference in the induction of remission with probiotics when compared to 5-ASA (RR 0.92, 95% CI 0.73 to 1.16; 1 study, 116 participants; low-certainty evidence; downgraded due to risk of bias and imprecision). There may be little or no difference in minor adverse events, but the evidence is of very low certainty (RR 1.33, 95% CI 0.53 to 3.33; 1 study, 116 participants). Reported adverse events included abdominal pain, nausea, headache and mouth ulcers. There were no serious adverse events with probiotics, however perforated sigmoid diverticulum and respiratory failure in a patient with severe emphysema were reported in the 5-ASA arm (RR 0.21, 95% CI 0.01 to 4.22; 1 study, 116 participants; very low-certainty evidence). Probiotics combined with 5-ASA versus 5-ASA alone Low-certainty evidence from a single study shows that when combined with 5-ASA, probiotics may slightly improve the induction of remission (based on the Sunderland disease activity index) compared to 5-ASA alone (RR 1.22 CI 1.01 to 1.47; 1 study, 84 participants; low-certainty evidence; downgraded due to unclear risk of bias and imprecision). No information about adverse events was reported. Time to remission, histological and biochemical outcomes were sparsely reported in the studies. None of the other secondary outcomes (progression to surgery, need for additional therapy, quality of life scores, or steroid withdrawal) were reported in any of the studies.
Authors' conclusions: Low-certainty evidence suggests that probiotics may induce clinical remission in active ulcerative colitis when compared to placebo. There may be little or no difference in clinical remission with probiotics alone compared to 5-ASA. There is limited evidence from a single study which failed to provide a definition of remission, that probiotics may slightly improve the induction of remission when used in combination with 5-ASA. There was no evidence to assess whether probiotics are effective in people with severe and more extensive disease, or if specific preparations are superior to others. Further targeted and appropriately designed RCTs are needed to address the gaps in the evidence base. In particular, appropriate powering of studies and the use of standardised participant groups and outcome measures in line with the wider field are needed, as well as reporting to minimise risk of bias.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Lakhbir Kaur: none known
Morris Gordon: received travel grants from various companies to attend scientific meetings in the last three years, including Biogaia, Synergy, Tillots, Ferring and Allergan. None of these companies have had any involvement in the planning, completion, analysis or write up of this or any other reviews. This review has been completed as part of a UK funded National Institute for Health Research (NIHR) Cochrane Programme grant, with some time funded.
Patricia Baines: none known
Zipporah Iheozor‐Ejiofor: my employment at the University of Central Lancashire is funded by the National Institute for Health Research (NIHR) UK and focuses on high‐priority Cochrane Reviews in inflammatory bowel disease.
Vasilliki Sinopoulou: none known
Anthony Akobeng: none known
Figures


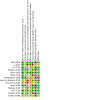









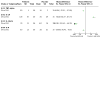


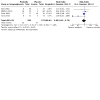







Update of
-
Probiotics for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2007 Oct 17;(4):CD005573. doi: 10.1002/14651858.CD005573.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2020 Mar 4;3:CD005573. doi: 10.1002/14651858.CD005573.pub3. PMID: 17943867 Updated.
References
References to studies included in this review
Kato 2004 {published data only}
-
- Kato K, Mizuno S, Umesaki Y, Ishii Y, Sugitani M, Imaoka A, et al. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Alimentary Pharmacology & Therapeutics 2004;20(10):1133-41. - PubMed
Li 2013 {published data only}
-
- Li K, Zhang CF, Xia YH, Li Z J, Han Y. Efficacy of probiotics on ulcerative colitis and it's mechanism. Zhonghua Weichang Waike Zazhi 2013;16(4):336-9. - PubMed
Liu 2014 {published data only}
-
- Liu P, Sun L, Zhang ZH, Zhang P, Zhang J. Clinical efficacy of Salofalk combined with beneficial bacteria in patients with ulcerative colitis. World Chinese Journal of Digestology 2014;22:3344-8.
Matthes 2010 {published data only}
Miele 2009 {published data only}
-
- Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. American Journal of Gastroenterology 2009;104(2):437-43. - PubMed
Oliva 2011 {published data only}
-
- Olivia S, Di Nardo G, Aloi M, Falconieri P, Ferrari F, Mallardo S, et al. Rectal infusion of lactobacillus reuteri ATCC 55730 improves inflammation and down regulates mucosal pro-inflammatory cytokines in distal ulcerative colitis. Journal of Paediatric Gastroenterology and Nutrition 2011;52:E156.
Rembacken 1999 {published data only}
-
- Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Eshcherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomized trial. Lancet 1999;354(9179):635-9. - PubMed
Sanchez‐Morales 2019 {published data only}
-
- Sánchez-Morales A, Pérez-Ayala MF, Cruz-Martínez M, Arenas-Osuna J, Ramírez-Mendoza P, Ceniceros RA, et al. Probiotics’ effectiveness on symptoms, histological features and feeding tolerance in ulcerative colitis [Efectividad de probióticos sobre síntomas, histología y tolerancia alimentaria en colitis ulcerativa]. Revista Medica del Instituto Mexicano del Seguro Social 2019;57(1):9-14. - PubMed
Solovyeva 2014 {published data only}
-
- Solovyeva O. Probiotics can extend the remission of ulcerative colitis. Journal of Crohn's and Colitis 2014;8:S221.
Sood 2009 {published data only}
-
- Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, et al. The probiotic preparation VSL#3 induces remission in patients with mild-moderate active ulcerative colitis. Clinical Gastroenterology and Hepatology 2009;7(22):1202-9. - PubMed
Tamaki 2016 {published data only}
-
- Tamaki H, Nakase H, Inoue S, Kawanami C, Itani T, Ohana M, et al. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: A randomized, double-blinded, placebo-controlled multicenter trial. Digestive Endoscopy 2016;28(1):67-74. - PubMed
Tursi 2010 {published data only}
-
- Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. American Journal of Gastroenterology 2010;105(10):2218-27. - PMC - PubMed
Vejdani 2017 {published data only}
-
- Vejdani R, Bahari A, Zadeh AM, Azmi M, Ebrahimi-Daryani N, Hashtroudi AA, et al. Effects of lactobacillus casei probiotic on mild to moderate ulcerative colitis: A placebo controlled study. Indian Journal of Medical Sciences 2017;69(1):24-8.
Zhang 2018a {published data only}
-
- Zhang J. Effects of Bifidobacterium quadruplex live bacteria tablets on Mayo score and hs-CRP, IL-4, IL-8 in patients with mild to moderate ulcerative colitis. World Chinese Journal of Digestology 2018;26(6):373-7.
References to studies excluded from this review
Ballini 2019 {published data only}
-
- Ballini A, Santacroce L, Cantore S, Bottalico L, Dipalma G, Topi S, et al. Probiotics efficacy on oxidative stress values in inflammatory bowel disease: a randomized double-blinded placebo-controlled pilot study. Endocrine, Metabolic & Immune Disorders - Drug Targets 2019;19(3):373-81. - PubMed
Bataga 2015 {published data only}
-
- Bataga SM, Torok I, Macarie M, Negovan A, Botianu A. Rifaximine and probiotics in the treatment of mild relapse of left sided ulcerative colitis. European Journal of Clinical Investigation 2015;45:39-40.
Bjarnason 2019 {published data only}
Fujimori 2009 {published data only}
-
- Fujimori S, Gudis K, Mitsui K, Seo T, Yonezawa M, Tanaka S, et al. A randomized controlled trial on the efficacy of symbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis. Nutrition 2009;25(5):520-5. - PubMed
IRCT20120415009475N5 {published data only}
-
- IRCT20120415009475N5. Evaluation of the effects of lyophilized Saccharomyces Boulardii capsules on quality of life and clinical outcomes in children with inflammatory bowel disease (IBD) [Evaluation of the effects of lyophilized Saccharomyces Boulardii capsules on quality of life and clinical outcomes in children with inflammatory bowel disease (IBD)]. www.irct.ir/trial/32179 Registration date 10 October 2018.
Ishikawa 2003 {published data only}
-
- Ishikawa H, Akedo I, Umesaki Y, Tanaka R, Imaoka, Otani T. Randomized controlled trial of the effect of bifidobacteria-fermented milk on ulcerative colitis. Journal of the American College of Nutrition 2003;22(1):56-63. - PubMed
Krag 2013 {published data only}
-
- Krag A, Munkholm P, Israelsen H, Ryberg B, Andersen KK, Bendtsen F. Profermin is efficacious in patients with active ulcerative colitis - a randomized controlled trial. Inflammatory Bowel Disease 2013;19(12):2584-92. - PubMed
NCT00374725 {published data only}
-
- NCT00374725. Treatment of ulcerative colitis with a combination of lactobacillus rhamnosus and lactobacillus acidophilus. www.clinicaltrials.gov/ct2/show/NCT00374725 (first received 11 September 2006).
NCT00895336 {published data only}
-
- NCT00895336. Lactobacillus GG in pediatric ulcerative colitis. clinicaltrials.gov/show/NCT00895336 (first received 8 May 2009).
Santana 2010 {published data only}
-
- Santana Porben S. Influence of a combination of lactobacilli and bifidobacteria upon disease activity, stool pattern and nutritional status of ulcerative colitis patients. Spanish society of parenteral and enteral nutrition 2010;25(6):971-83. - PubMed
Turcotte 2011 {published data only}
-
- Turcotte JF, Huynh HQ. Treatment with the probiotic VSL#3 as an adjunctive therapy in relapsing mild-to-moderate ulcerative colitis significantly reduces ulcerative colitis disease activity. Evidence-Based Medicine 2011;16(4):108-9. - PubMed
References to studies awaiting assessment
Fan 2019 {published data only}
Fang 2019 {published data only}
-
- Fang W, Qingfeng C. Effect of mesalazine combined with bifid on inflammatory response and rectal-anal dynamics in patients with ulcerative colitis. World Chinese Journal of Digestology 2018;26(10):594-600.
Huang 2018 {published data only}
-
- Huang M, Chen Z, Lang C, Chen J, Yang B, Xue L, et al. Efficacy of mesalazine in combination with bifid triple viable capsules on ulcerative colitis and the resultant effect on the inflammatory factors. Pakistan Journal of Pharmaceutical Sciences 2018;31(6):2891-5. - PubMed
Shi 2018 {published data only}
-
- Shi XH, Tan FP, Jiang WH. Bacillus subtilis and Enterococcus faecium enteric-coated capsules combined with mesalazine for treatment of patients with ulcerative colitis: Efficacy and impact on serum levels of SOD, MDA, interleukins, and TNF-α. World Chinese Journal of Digestology 2018;26(12):748-54.
Yilmaz 2019 {published data only}
Zhang 2018b {published data only}
-
- Zhang Y, Wu M, Chen Y. Effects of adjuvant therapy with bifidobacterium quadruplex on lipid peroxidation injury indexes, inflammatory factors and immune function in patients with ulcerative colitis. World Chinese Journal of Digestology 2018;26(22):1348-54.
References to ongoing studies
NCT04006977 {published data only}
-
- NCT04006977. Multistrain Probiotics Reduces UC Depression and Anxiety Scores. clinicaltrials.gov/ct2/show/NCT04006977 First posted 5 July 2019.
Additional references
Cohen 2010
-
- Cohen RD. Systematic review: the costs of ulcerative colitis in Western countries. Alimentary Pharmaology and Therapeutics 2010;31(7):693-707. - PubMed
Covidence [Computer program]
-
- Veritas Health Innovation Covidence. Version accessed prior to 4 February 2020. Melbourne, Australia: Veritas Health Innovation.Available at covidence.org.
Cui 2004
Dieleman 2003
Egger 1997
Fabia 1993
-
- Fabia R, Ar'Rajab A, Johannsson ML, Willen R, Andersson R, Molin G, et al. The effect of exogenous administration of Lactobacillus reuteri R2LC and oat fiber on acetic acid-induced colitis in the rat. Scandinavian Journal of Gastroenterology 1993;28(2):155-62. - PubMed
Fiorino 2016
GRADEpro GDT 2015 [Computer program]
-
- McMaster University GRADEpro GDT: GRADEpro Guideline Development Tool. McMaster University, 2015 (developed by Evidence Prime, Inc.).Available from gradepro.org.
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kennedy 2000
-
- Kennedy RJ, Hoper M, Deodhar K, Kirk SJ, Gardiner KR. Probiotic therapy fails to improve gut permeability in a hapten model of colitis. Scandinavian Journal of Gastroenterology 2000;35(12):1266-71. - PubMed
Kruis 1997
-
- Kruis W, Schutz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Alimentary Pharmacology and Therapeutics 1997;11(5):853-8. - PubMed
Kruis 2004
Loftus 2004
-
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence and environmental influences. Gastroenterology 2004;126(6):1504-17. [PMID: ] - PubMed
Madsen 1999
-
- Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology 1999;116(5):1107-14. - PubMed
Madsen 2001
-
- Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001;121(3):580-91. - PubMed
NICE 2013
-
- National Institute for Health and Care Excellence. Ulcerative Colitis Mangagement in adults, children and Young people CG166. NICE 2013. [https://www.nice.org.uk/guidance/ng130/evidence/june-2013-full-guideline...]
Ong 2019
Panigrahi 2014
-
- Panigrahi P. Probiotics and prebiotics in neonatal necrotizing enterocolitis: New opportunities for translational research. Pathophysiology 2014;21(1):35-46. - PubMed
Pitkin 1999
-
- Pitkin RM, Branagan MA, Burmeister LF. Accuracy of data in abstracts of published research articles. JAMA 1999;281:1110-1. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schultz 2002
-
- Schultz M, Veltkamp C, Dieleman LA, Grenther WB, Wyrick PB, Tonkonogy SL, et al. Lactobacillus plantarum 299V in the treatment and prevention of spontaneous colitis in interleukin-10-deficient mice. Inflammatory Bowel Diseases 2002;8(2):71-80. - PubMed
Schünemann 2011
-
- Schünemann H, Hill S, Guyatt G, et al. The GRADE approach and Bradford Hill's criteria for causation. Journal of Epidemiology & Community Health 2011;65:392-5. - PubMed
Shibolet 2002
-
- Shibolet O, Karmeli F, Eliakim R, Swennen E, Brigidi P, Gionchetti P, et al. Variable response to probiotics in two models of experimental colitis in rats. Inflammatory Bowel Diseases 2002;8(6):399-406. - PubMed
Truelove 1955
Turner 2018
-
- Turner D, Ruemmele FM, Orlanski-Meyer E, Griffiths AM, Carpi JM, Bronsky J, et al. Management of paediatric ulcerative colitis, part 1: ambulatory care—an evidence-based guideline from European Crohn's and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2018;62(2):257-91. [DOI: 10.1097/mpg.0000000000002035] - DOI - PubMed
Ungaro 2016
Zocco 2006
-
- Zocco MA, dal Verme LZ, Cremonini F, Piscaglia AC, Nista EC, Candelli M, et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Alimentary Pharmacology and Therapeutics 2006;23(11):1567-74. - PubMed
References to other published versions of this review
Mallon 2005
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

