Metformin Reduces Aging-Related Leaky Gut and Improves Cognitive Function by Beneficially Modulating Gut Microbiome/Goblet Cell/Mucin Axis
- PMID: 32129462
- PMCID: PMC7302182
- DOI: 10.1093/gerona/glaa056
Metformin Reduces Aging-Related Leaky Gut and Improves Cognitive Function by Beneficially Modulating Gut Microbiome/Goblet Cell/Mucin Axis
Abstract
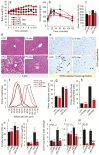
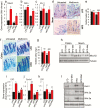
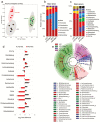
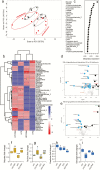
Aging-related illnesses are increasing and effective strategies to prevent and/or treat them are lacking. This is because of a poor understanding of therapeutic targets. Low-grade inflammation is often higher in older adults and remains a key risk factor of aging-related morbidities and mortalities. Emerging evidence indicates that abnormal (dysbiotic) gut microbiome and dysfunctional gut permeability (leaky gut) are linked with increased inflammation in older adults. However, currently available drugs do not treat aging-related microbiome dysbiosis and leaky gut, and little is known about the cellular and molecular processes that can be targeted to reduce leaky gut in older adults. Here, we demonstrated that metformin, a safe Food and Drug Administration-approved antidiabetic drug, decreased leaky gut and inflammation in high-fat diet-fed older obese mice, by beneficially modulating the gut microbiota. In addition, metformin increased goblet cell mass and mucin production in the obese older gut, thereby decreasing leaky gut and inflammation. Mechanistically, metformin increased the goblet cell differentiation markers by suppressing Wnt signaling. Our results suggest that metformin can be used as a regimen to prevent and treat aging-related leaky gut and inflammation, especially in obese individuals and people with western-style high-fat dietary lifestyle, by beneficially modulating gut microbiome/goblet cell/mucin biology.
Keywords: Gut permeability; Inflammation; Microbiota; Mucus; Wnt signaling.
© The Author(s) 2020. Published by Oxford University Press on behalf of The Gerontological Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Stehle JR Jr, Leng X, Kitzman DW, Nicklas BJ, Kritchevsky SB, High KP. Lipopolysaccharide-binding protein, a surrogate marker of microbial translocation, is associated with physical function in healthy older adults. J Gerontol A Biol Sci Med Sci. 2012;67:1212–1218. doi: 10.1093/gerona/gls178 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK103531/DK/NIDDK NIH HHS/United States
- I01 BX005109/BX/BLRD VA/United States
- R01 AG018915/AG/NIA NIH HHS/United States
- R01 AG052419/AG/NIA NIH HHS/United States
- K01 AG059837/AG/NIA NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- R01 AG059416/AG/NIA NIH HHS/United States
- P30 AG021332/AG/NIA NIH HHS/United States
- U01 AG060897/AG/NIA NIH HHS/United States
- U13 AG040938/AG/NIA NIH HHS/United States
- U24 AG059624/AG/NIA NIH HHS/United States
- KL2 TR001421/TR/NCATS NIH HHS/United States
- R01 AG045551/AG/NIA NIH HHS/United States
- R01 DK081842/DK/NIDDK NIH HHS/United States
- U24 AG058556/AG/NIA NIH HHS/United States
- RF1 AG054474/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

