Fedratinib in patients with myelofibrosis previously treated with ruxolitinib: An updated analysis of the JAKARTA2 study using stringent criteria for ruxolitinib failure
- PMID: 32129512
- PMCID: PMC7317815
- DOI: 10.1002/ajh.25777
Fedratinib in patients with myelofibrosis previously treated with ruxolitinib: An updated analysis of the JAKARTA2 study using stringent criteria for ruxolitinib failure
Abstract
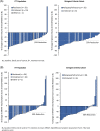
Fedratinib is an oral, selective Janus kinase 2 (JAK2) inhibitor. The phase II JAKARTA2 study assessed fedratinib in patients with intermediate- or high-risk myelofibrosis (MF) who were resistant or intolerant to prior ruxolitinib per investigator assessment. Patients received fedratinib 400 mg/day in 28-day cycles. The JAKARTA2 outcomes were initially reported using a last-observation-carried forward (LOCF) analysis in a "Per Protocol" population. This updated analysis of JAKARTA2 employs intention-to-treat analysis principles without LOCF for all treated patients (ITT Population; N = 97), and for a patient subgroup who met more stringent definitions of prior ruxolitinib failure (Stringent Criteria Cohort; n = 79). Median duration of prior ruxolitinib exposure was 10.7 months. The primary endpoint was spleen volume response rate (SVRR; ≥35% spleen volume decrease from baseline to end of cycle 6 [EOC6]). The SVRR was 31% in the ITT Population and 30% in the Stringent Criteria Cohort. Median duration of spleen volume response was not reached. Symptom response rate (≥50% reduction from baseline to EOC6 in total symptom score [TSS] on the modified Myelofibrosis Symptom Assessment Form [MFSAF]) was 27%. Grade 3-4 anemia and thrombocytopenia rates were 38% and 22%, respectively. Patients with advanced MF substantially pretreated with ruxolitinib attained robust spleen responses and reduced symptom burden with fedratinib.
© 2020 The Authors. American Journal of Hematology published by Wiley Periodicals, Inc.
Figures
References
-
- National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology ‐ Myeloproliferative Neoplasms Version 3.2019. 2019. https://www.nccn.org/patients/guidelines/content/PDF/mpn-patient.pdf. Accessed August 23, 2019.
-
- Tefferi A. Primary myelofibrosis: 2017 update on diagnosis, risk‐stratification, and management. Am J Hematol. 2016;91(12):1262‐1271. - PubMed
-
- Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29(4):392‐397. - PubMed
-
- Mascarenhas J. Looking forward: novel therapeutic approaches in chronic and advanced phases of myelofibrosis. Hematology Am Soc Hematol Educ Program. 2015;2015:329‐339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous