Nickel-Catalyzed Alkyl-Alkyl Cross-Electrophile Coupling Reaction of 1,3-Dimesylates for the Synthesis of Alkylcyclopropanes
- PMID: 32129601
- PMCID: PMC7864534
- DOI: 10.1021/jacs.0c01330
Nickel-Catalyzed Alkyl-Alkyl Cross-Electrophile Coupling Reaction of 1,3-Dimesylates for the Synthesis of Alkylcyclopropanes
Abstract
Cross-electrophile coupling reactions of two Csp3-X bonds remain challenging. Herein we report an intramolecular nickel-catalyzed cross-electrophile coupling reaction of 1,3-diol derivatives. Notably, this transformation is utilized to synthesize a range of mono- and 1,2-disubstituted alkylcyclopropanes, including those derived from terpenes, steroids, and aldol products. Additionally, enantioenriched cyclopropanes are synthesized from the products of proline-catalyzed and Evans aldol reactions. A procedure for direct transformation of 1,3-diols to cyclopropanes is also described. Calculations and experimental data are consistent with a nickel-catalyzed mechanism that begins with stereoablative oxidative addition at the secondary center.
Figures
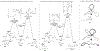
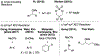

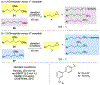

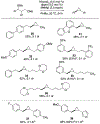
References
-
- Biswas S; Weix DJ Mechanism and Selectivity in Nickel-Catalyzed Cross-Electrophile Coupling of Aryl Halides with Alkyl Halides. J. Am. Chem. Soc 2013, 135, 16192–16197. - PMC - PubMed
- Everson DA; Weix DJ Cross-Electrophile Coupling: Principles of Reactivity and Selectivity. J. Org. Chem 2014, 79, 4793–4798. - PMC - PubMed
- Weix DJ Methods and Mechanisms for Cross-Electrophile Coupling of Csp2 Halides with Alkyl Electrophiles. Acc. Chem. Res 2015, 48, 1767–1775. - PMC - PubMed
- Knappke CEI; Grupe S; Gärtner D; Corpet M; Gosmini C; Jacobi von Wangelin A Reductive Cross-Coupling Reactions between Two Electrophiles. Chem. Eur. J 2014, 20, 6828–6842. - PubMed
- Wang X; Dai Y; Gong H Nickel-Catalyzed Reductive Couplings. Top. Curr. Chem 2016, 374, 43. - PubMed
-
- Lucas EL; Jarvo ER Stereospecific and Stereoconvergent Cross-Couplings Between Alkyl Electrophiles. Nat. Rev. Chem 2017, 1, 0065.
-
- Tollefson EJ; Erickson LW Jarvo ER Stereospecific Intramolecular Reductive Cross-Electrophile Coupling Reactions for Cyclopropane Synthesis. J. Am. Chem. Soc 2015, 137, 9760. - PubMed
- Erickson LW Lucas EL; Tollefson EJ; Jarvo ER Nickel-Catalyzed Cross-Electrophile Coupling of Alkyl Fluorides: Stereospecific Synthesis of Vinylcyclopropanes. J. Am. Chem. Soc 2016, 138, 14006–14011. - PubMed
- Konev MO; Hanna LE; Jarvo ER Intra- and Intermolecular Nickel-Catalyzed Reductive Cross-Electrophile Coupling Reactions of Benzylic Esters with Aryl Halides. Angew. Chem. Int. Ed 2006, 55, 6730–6733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

