In Vitro Metabolism of Isopropylated and tert-Butylated Triarylphosphate Esters Using Human Liver Subcellular Fractions
- PMID: 32129605
- PMCID: PMC7508416
- DOI: 10.1021/acs.chemrestox.0c00002
In Vitro Metabolism of Isopropylated and tert-Butylated Triarylphosphate Esters Using Human Liver Subcellular Fractions
Abstract
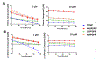
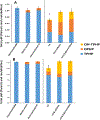
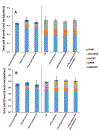
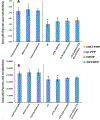
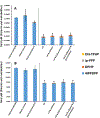
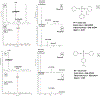
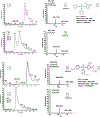
Isopropylated and tert-butylated triarylphosphate esters (ITPs and TBPPs, respectively) are plasticizers and flame retardants that are ubiquitous in indoor environments; however, no studies to date have characterized their metabolism. Using human liver subcellular S9 fractions, phase I and II in vitro metabolism of triphenyl phosphate (TPHP), 4-tert-butylphenyl diphenyl phosphate (4tBPDPP), 2-isopropylphenyl diphenyl phosphate (2IPPDPP), and 4-isopropylphenyl diphenyl phosphate (4IPPDPP) was investigated at 1 and 10 μM doses. Parent depletion and the formation of known or suspected metabolites (e.g., likely hydrolysis or hydroxylated products), including diphenyl phosphate (DPHP), hydroxyl-triphenyl phosphate (OH-TPHP), isopropylphenyl phenyl phosphate (ip-PPP), and tert-butylphenyl phenyl phosphate (tb-PPP), were monitored and quantified via GC/MS or LC-MS/MS. tb-PPP and its conjugates were identified as the major in vitro metabolites of 4tBPDPP and accounted for 71% and 49%, respectively, of the parent molecule that was metabolized during the incubation. While the mass balance between parents and metabolites was conserved for TPHP and 4tBPDPP, approximately 20% of the initial parent mass was unaccounted for after quantifying suspected metabolites of 2IPPDPP and 4IPPDPP that had authentic standards available. Two novel ITP metabolites, mono-isopropenylphenyl diphenyl phosphate and hydroxy-isopropylphenyl diphenyl phosphate, were tentatively identified by high-resolution mass spectrometry and screened for in recently collected human urine where mono-isopropenylphenyl diphenyl phosphate was detected in one of nine samples analyzed. This study provides insight into the biological fate of ITP and TBPP isomers in human tissues and is useful in identifying appropriate biomarkers of exposure to monitor, particularly in support of epidemiological studies.
Figures
References
-
- Weil ED; Levchik S; Moy P Flame and Smoke Retardants in Vinyl Chloride Polymers – Commercial Usage and Current Developments. J. Fire Sci 2016, 24 (3), 211–236.
-
- Cooper EM; Kroeger G; Davis K; Clark CR; Ferguson PL; Stapleton HM Results from Screening Polyurethane Foam Based Consumer Products for Flame Retardant Chemicals: Assessing Impacts on the Change in the Furniture Flammability Standards. Environ. Sci. Technol 2016, 50 (19), 10653–10660. - PMC - PubMed
-
- Phillips AL; Hammel SC; Konstantinov A; Stapleton HM Characterization of Individual Isopropylated and tert-Butylated Triarylphosphate (ITP and TBPP) Isomers in Several Commercial Flame Retardant Mixtures and House Dust Standard Reference Material SRM 2585. Environ. Sci. Technol 2017, 51 (22), 13443–13449. - PMC - PubMed
-
- Alzualde A; Behl M; Sipes NS; Hsieh JH; Alday A; Tice RR; Paules RS; Muriana A; Quevedo C Toxicity profiling of flame retardants in zebrafish embryos using a battery of assays for developmental toxicity, neurotoxicity, cardiotoxicity and hepatotoxicity toward human relevance. Neurotoxicol. Teratol 2018, 70, 40–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

