Effect of a Mobile Phone-Based Glucose-Monitoring and Feedback System for Type 2 Diabetes Management in Multiple Primary Care Clinic Settings: Cluster Randomized Controlled Trial
- PMID: 32130172
- PMCID: PMC7066511
- DOI: 10.2196/16266
Effect of a Mobile Phone-Based Glucose-Monitoring and Feedback System for Type 2 Diabetes Management in Multiple Primary Care Clinic Settings: Cluster Randomized Controlled Trial
Abstract
Background: Recent evidence of the effectiveness of mobile phone-based diabetes management systems is generally based on studies conducted in tertiary hospitals or professional diabetes clinics.
Objective: This study aimed to evaluate the clinical efficacy and applicability of a mobile phone-based glucose-monitoring and feedback system for the management of type 2 diabetes mellitus (T2DM) in multiple primary care clinic settings.
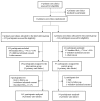
Methods: In this multicenter, cluster-randomized controlled, open trial, 13 primary care clinics in Seoul and other large cities in South Korea were voluntarily recruited. Overall, 150 (9 clinics) and 97 (4 clinics) participants with T2DM were assigned to the intervention and control groups, respectively (2:1 allocation). Every month, participants in both groups attended face-to-face physicians' consultation for the management of diabetes in the clinic. For the intervention group, participants were required to upload their daily self-monitoring of blood glucose (SMBG) results using the mobile phone app in addition to outpatient care for 3 months. The results were automatically transmitted to the main server. Physicians had to check their patients' SMBG results through an administrator's website and send a short feedback message at least once a week. At baseline and 3 months, both groups had anthropometry and blood tests, including hemoglobin A1c (HbA1c), and responded to questionnaires about treatment satisfaction and compliance.
Results: At 3 months, participants in the intervention group showed significantly more improvement in HbA1c (adjusted mean difference to control -0.30%, 95% CI -0.50 to -0.11; P=.003) and fasting plasma glucose (-17.29 mg/dL, 95% CI -29.33 to -5.26; P=.005) than those in the control group. In addition, there was significantly more reduction in blood pressure, and the score regarding treatment satisfaction and motivation for medication adherence increased more in the intervention group than in the control group. In the subgroup analyses, the effect on glycemic control was more significant among younger patients and higher baseline HbA1c levels.
Conclusions: The mobile phone-based glucose-monitoring and feedback system was effective in glycemic control when applied in primary care clinic settings. This system could be utilized effectively with diverse institutions and patients.
Trial registration: Clinical Research Information Service (CRIS) https://tinyurl.com/tgqawbz.
Keywords: diabetes mellitus, type 2; mHealth; primary care; telehealth.
©Yeoree Yang, Eun Young Lee, Hun-Sung Kim, Seung-Hwan Lee, Kun-Ho Yoon, Jae-Hyoung Cho. Originally published in JMIR mHealth and uHealth (http://mhealth.jmir.org), 26.02.2020.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures


References
-
- NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016 Apr 9;387(10027):1513–30. doi: 10.1016/S0140-6736(16)00618-8. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(16)00618-8 - DOI - PMC - PubMed
-
- Powers MA, Bardsley J, Cypress M, Duker P, Funnell MM, Fischl AH, Maryniuk MD, Siminerio L, Vivian E. Diabetes self-management education and support in type 2 diabetes: a joint position statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. Clin Diabetes. 2016 Apr;34(2):70–80. doi: 10.2337/diaclin.34.2.70. http://europepmc.org/abstract/MED/27092016 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

