Expedited Safety Reporting to Sponsors Through the Implementation of an Alert System for Clinical Trial Management at an Academic Medical Center: Retrospective Design Study
- PMID: 32130175
- PMCID: PMC7068534
- DOI: 10.2196/14379
Expedited Safety Reporting to Sponsors Through the Implementation of an Alert System for Clinical Trial Management at an Academic Medical Center: Retrospective Design Study
Abstract
Background: Early detection or notification of adverse event (AE) occurrences during clinical trials is essential to ensure patient safety. Clinical trials take advantage of innovative strategies, clinical designs, and state-of-the-art technologies to evaluate efficacy and safety, however, early awareness of AE occurrences by investigators still needs to be systematically improved.
Objective: This study aimed to build a system to promptly inform investigators when clinical trial participants make unscheduled visits to the emergency room or other departments within the hospital.
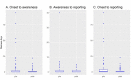
Methods: We developed the Adverse Event Awareness System (AEAS), which promptly informs investigators and study coordinators of AE occurrences by automatically sending text messages when study participants make unscheduled visits to the emergency department or other clinics at our center. We established the AEAS in July 2015 in the clinical trial management system. We compared the AE reporting timeline data of 305 AE occurrences from 74 clinical trials between the preinitiative period (December 2014-June 2015) and the postinitiative period (July 2015-June 2016) in terms of three AE awareness performance indicators: onset to awareness, awareness to reporting, and onset to reporting.
Results: A total of 305 initial AE reports from 74 clinical trials were included. All three AE awareness performance indicators were significantly lower in the postinitiative period. Specifically, the onset-to-reporting times were significantly shorter in the postinitiative period (median 1 day [IQR 0-1], mean rank 140.04 [SD 75.35]) than in the preinitiative period (median 1 day [IQR 0-4], mean rank 173.82 [SD 91.07], P≤.001). In the phase subgroup analysis, the awareness-to-reporting and onset-to-reporting indicators of phase 1 studies were significantly lower in the postinitiative than in the preinitiative period (preinitiative: median 1 day, mean rank of awareness to reporting 47.94, vs postinitiative: median 0 days, mean rank of awareness to reporting 35.75, P=.01; and preinitiative: median 1 day, mean rank of onset to reporting 47.4, vs postinitiative: median 1 day, mean rank of onset to reporting 35.99, P=.03). The risk-level subgroup analysis found that the onset-to-reporting time for low- and high-risk studies significantly decreased postinitiative (preinitiative: median 4 days, mean rank of low-risk studies 18.73, vs postinitiative: median 1 day, mean rank of low-risk studies 11.76, P=.02; and preinitiative: median 1 day, mean rank of high-risk studies 117.36, vs postinitiative: median 1 day, mean rank of high-risk studies 97.27, P=.01). In particular, onset to reporting was reduced more in the low-risk trial than in the high-risk trial (low-risk: median 4-0 days, vs high-risk: median 1-1 day).
Conclusions: We demonstrated that a real-time automatic alert system can effectively improve safety reporting timelines. The improvements were prominent in phase 1 and in low- and high-risk clinical trials. These findings suggest that an information technology-driven automatic alert system effectively improves safety reporting timelines, which may enhance patient safety.
Keywords: adverse event; clinical trial; early detection; patient safety.
©Yu Rang Park, HaYeong Koo, Young-Kwang Yoon, Sumi Park, Young-Suk Lim, Seunghee Baek, Hae Reong Kim, Tae Won Kim. Originally published in JMIR Medical Informatics (http://medinform.jmir.org), 23.02.2020.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
LinkOut - more resources
Full Text Sources

