Immunotherapy with DNA vaccine and live attenuated rubella/SIV gag vectors plus early ART can prevent SIVmac251 viral rebound in acutely infected rhesus macaques
- PMID: 32130229
- PMCID: PMC7055890
- DOI: 10.1371/journal.pone.0228163
Immunotherapy with DNA vaccine and live attenuated rubella/SIV gag vectors plus early ART can prevent SIVmac251 viral rebound in acutely infected rhesus macaques
Abstract
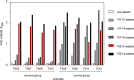
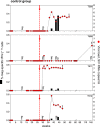
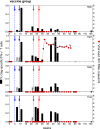
Anti-retroviral therapy (ART) has been highly successful in controlling HIV replication, reducing viral burden, and preventing both progression to AIDS and viral transmission. Yet, ART alone cannot cure the infection. Even after years of successful therapy, ART withdrawal leads inevitably to viral rebound within a few weeks or months. Our hypothesis: effective therapy must control both the replicating virus pool and the reactivatable latent viral reservoir. To do this, we have combined ART and immunotherapy to attack both viral pools simultaneously. The vaccine regimen consisted of DNA vaccine expressing SIV Gag, followed by a boost with live attenuated rubella/gag vectors. The vectors grow well in rhesus macaques, and they are potent immunogens when used in a prime and boost strategy. We infected rhesus macaques by high dose mucosal challenge with virulent SIVmac251 and waited three days to allow viral dissemination and establishment of a reactivatable viral reservoir before starting ART. While on ART, the control group received control DNA and empty rubella vaccine, while the immunotherapy group received DNA/gag prime, followed by boosts with rubella vectors expressing SIV gag over 27 weeks. Both groups had a vaccine "take" to rubella, and the vaccine group developed antibodies and T cells specific for Gag. Five weeks after the last immunization, we stopped ART and monitored virus rebound. All four control animals eventually had a viral rebound, and two were euthanized for AIDS. One control macaque did not rebound until 2 years after ART release. In contrast, there was only one viral rebound in the vaccine group. Three out of four vaccinees had no viral rebound, even after CD8 depletion, and they remain in drug-free viral remission more than 2.5 years later. The strategy of early ART combined with immunotherapy can produce a sustained SIV remission in macaques and may be relevant for immunotherapy of HIV in humans.
Conflict of interest statement
The authors have read the journal's policy and declare the following potential competing interests: KEB was employed by a commercial company, Inovio Pharmaceuticals, Inc. and ML was employed by BioQual, Inc. This does not alter our adherence to PLOS ONE policies on sharing data and materials. There are no patents, products in development or marketed products to declare.
Figures


References
-
- Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS pathogens. 2013;9(3):e1003211 10.1371/journal.ppat.1003211 - DOI - PMC - PubMed
-
- Sneller MC, Justement JS, Gittens KR, Petrone ME, Clarridge KE, Proschan MA, et al. A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection. Sci Transl Med. 2017;9(419). 10.1126/scitranslmed.aan8848 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

