Meropenem vs standard of care for treatment of neonatal late onset sepsis (NeoMero1): A randomised controlled trial
- PMID: 32130261
- PMCID: PMC7055900
- DOI: 10.1371/journal.pone.0229380
Meropenem vs standard of care for treatment of neonatal late onset sepsis (NeoMero1): A randomised controlled trial
Abstract
Background: The early use of broad-spectrum antibiotics remains the cornerstone for the treatment of neonatal late onset sepsis (LOS). However, which antibiotics should be used is still debatable, as relevant studies were conducted more than 20 years ago, recruited in single centres or countries, evaluated antibiotics not in clinical use anymore and had variable inclusion/exclusion criteria and outcome measures. Moreover, antibiotic-resistant bacteria have become a major problem in many countries worldwide. We hypothesized that efficacy of meropenem as a broad-spectrum antibiotic is superior to standard of care regimens (SOC) in empiric treatment of LOS and aimed to compare meropenem to SOC in infants aged <90 days with LOS.
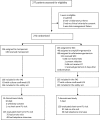
Methods and findings: NeoMero-1 was a randomized, open-label, phase III superiority trial conducted in 18 neonatal units in 6 countries. Infants with post-menstrual age (PMA) of ≤44 weeks with positive blood culture and one, or those with negative culture and at least with two predefined clinical and laboratory signs suggestive of LOS, or those with PMA >44 weeks meeting the Goldstein criteria of sepsis, were randomized in a 1:1 ratio to receive meropenem or one of the two SOC regimens (ampicillin+gentamicin or cefotaxime+gentamicin) chosen by each site prior to the start of the study for 8-14 days. The primary outcome was treatment success (survival, no modification of allocated therapy, resolution/improvement of clinical and laboratory markers, no need of additional antibiotics and presumed/confirmed eradication of pathogens) at test-of-cure visit (TOC) in full analysis set. Stool samples were tested at baseline and Day 28 for meropenem-resistant Gram-negative organisms (CRGNO). The primary analysis was performed in all randomised patients and in patients with culture confirmed LOS. Proportions of participants with successful outcome were compared by using a logistic regression model adjusted for the stratification factors. From September 3, 2012 to November 30th 2014, total of 136 patients (instead of planned 275) in each arm were randomized; 140 (52%) were culture positive. Successful outcome at TOC was achieved in 44/136 (32%) in the meropenem arm vs. 31/135 (23%) in the SOC arm (p = 0.087). The respective numbers in patients with positive cultures were 17/63 (27%) vs. 10/77 (13%) (p = 0.022). The main reason of failure was modification of allocated therapy. Treatment emergent adverse events occurred in 72% and serious adverse events in 17% of patients, the Day 28 mortality was 6%. Cumulative acquisition of CRGNO by Day 28 occurred in 4% of patients in the meropenem and 12% in the SOC arm (p = 0.052).
Conclusions: Within this study population, we found no evidence that meropenem was superior to SOC in terms of success at TOC, short term hearing disturbances, safety or mortality were similar in both treatment arms but the study was underpowered to detect the planned effect. Meropenem treatment did not select for colonization with CRGNOs. We suggest that meropenem as broad-spectrum antibiotic should be reserved for neonates who are more likely to have Gram-negative LOS, especially in NICUs where microorganisms producing extended spectrum- and AmpC type beta-lactamases are circulating.
Conflict of interest statement
We declare the following competing interests: Chiesi Farmaceutici S.P.A. provided meropenem and collaborated in safety reporting but had no role in the study design or data analysis. This does not alter our adherence to PLOS ONE policies on sharing data and materials. There is no ohter competing interest to declare.
Figures