Host-glycan metabolism is regulated by a species-conserved two-component system in Streptococcus pneumoniae
- PMID: 32130269
- PMCID: PMC7075642
- DOI: 10.1371/journal.ppat.1008332
Host-glycan metabolism is regulated by a species-conserved two-component system in Streptococcus pneumoniae
Abstract
Pathogens of the Streptococcus genus inhabit many different environmental niches during the course of an infection in a human host and the bacteria must adjust their metabolism according to available nutrients. Despite their lack of the citric-acid cycle, some streptococci proliferate in niches devoid of a readily available carbohydrate source. Instead they rely on carbohydrate scavenging for energy acquisition, which are obtained from the host. Here we discover a two-component system (TCS07) of Streptococcus pneumoniae that responds to glycoconjugated structures on proteins present on the host cells. Using next-generation RNA sequencing we find that the uncharacterized TCS07 regulon encodes proteins important for host-glycan processing and transporters of the released glycans, as well as intracellular carbohydrate catabolizing enzymes. We find that a functional TCS07 allele is required for growth on the glycoconjugated model protein fetuin. Consistently, we see a TCS07-dependent activation of the glycan degradation pathway. Thus, we pinpoint the molecular constituents responsible for sensing host derived glycans and link this to the induction of the proteins necessary for glycan degradation. Furthermore, we connect the TCS07 regulon to virulence in a mouse model, thereby establishing that host-derived glycan-metabolism is important for infection in vivo. Finally, a comparative phylogenomic analysis of strains from the Streptococcus genus reveal that TCS07 and most of its regulon is specifically conserved in species that utilize host-glycans for growth.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
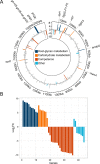
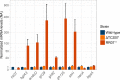
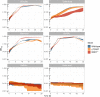
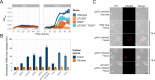
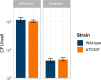
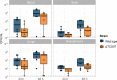
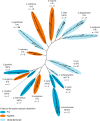


References
-
- Peterson SN, Sung CK, Cline R, Desai B V, Snesrud EC, Luo P, et al. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol Microbiol. 2004;51: 1051–70. Available: http://www.ncbi.nlm.nih.gov/pubmed/14763980 10.1046/j.1365-2958.2003.03907.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

