JC Virus infected choroid plexus epithelial cells produce extracellular vesicles that infect glial cells independently of the virus attachment receptor
- PMID: 32130281
- PMCID: PMC7075641
- DOI: 10.1371/journal.ppat.1008371
JC Virus infected choroid plexus epithelial cells produce extracellular vesicles that infect glial cells independently of the virus attachment receptor
Abstract
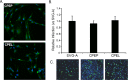
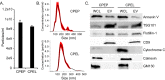
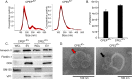
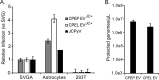
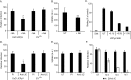
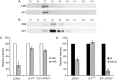
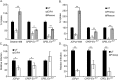
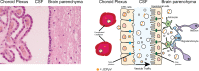
The human polyomavirus, JCPyV, is the causative agent of progressive multifocal leukoencephalopathy (PML) in immunosuppressed and immunomodulated patients. Initial infection with JCPyV is common and the virus establishes a long-term persistent infection in the urogenital system of 50-70% of the human population worldwide. A major gap in the field is that we do not know how the virus traffics from the periphery to the brain to cause disease. Our recent discovery that human choroid plexus epithelial cells are fully susceptible to virus infection together with reports of JCPyV infection of choroid plexus in vivo has led us to hypothesize that the choroid plexus plays a fundamental role in this process. The choroid plexus is known to relay information between the blood and the brain by the release of extracellular vesicles. This is particularly important because human macroglia (oligodendrocytes and astrocytes), the major targets of virus infection in the central nervous system (CNS), do not express the known attachment receptors for the virus and do not bind virus in human tissue sections. In this report we show that JCPyV infected choroid plexus epithelial cells produce extracellular vesicles that contain JCPyV and readily transmit the infection to human glial cells. Transmission of the virus by extracellular vesicles is independent of the known virus attachment receptors and is not neutralized by antisera directed at the virus. We also show that extracellular vesicles containing virus are taken into target glial cells by both clathrin dependent endocytosis and macropinocytosis. Our data support the hypothesis that the choroid plexus plays a fundamental role in the dissemination of virus to brain parenchyma.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Highly restrictive and directional penetration of the blood cerebral spinal fluid barrier by JCPyV.PLoS Pathog. 2024 Jul 22;20(7):e1012335. doi: 10.1371/journal.ppat.1012335. eCollection 2024 Jul. PLoS Pathog. 2024. PMID: 39038049 Free PMC article.
-
Complexities of JC Polyomavirus Receptor-Dependent and -Independent Mechanisms of Infection.Viruses. 2022 May 24;14(6):1130. doi: 10.3390/v14061130. Viruses. 2022. PMID: 35746603 Free PMC article. Review.
-
Susceptibility of Primary Human Choroid Plexus Epithelial Cells and Meningeal Cells to Infection by JC Virus.J Virol. 2018 Mar 28;92(8):e00105-18. doi: 10.1128/JVI.00105-18. Print 2018 Apr 15. J Virol. 2018. PMID: 29437972 Free PMC article.
-
Teriflunomide Inhibits JCPyV Infection and Spread in Glial Cells and Choroid Plexus Epithelial Cells.Int J Mol Sci. 2021 Sep 10;22(18):9809. doi: 10.3390/ijms22189809. Int J Mol Sci. 2021. PMID: 34575975 Free PMC article.
-
JC polyomavirus attachment, entry, and trafficking: unlocking the keys to a fatal infection.J Neurovirol. 2015 Dec;21(6):601-13. doi: 10.1007/s13365-014-0272-4. Epub 2014 Jul 31. J Neurovirol. 2015. PMID: 25078361 Free PMC article. Review.
Cited by
-
The choroid plexus and its role in the pathogenesis of neurological infections.Fluids Barriers CNS. 2022 Sep 10;19(1):75. doi: 10.1186/s12987-022-00372-6. Fluids Barriers CNS. 2022. PMID: 36088417 Free PMC article. Review.
-
Highly restrictive and directional penetration of the blood cerebral spinal fluid barrier by JCPyV.PLoS Pathog. 2024 Jul 22;20(7):e1012335. doi: 10.1371/journal.ppat.1012335. eCollection 2024 Jul. PLoS Pathog. 2024. PMID: 39038049 Free PMC article.
-
Complexities of JC Polyomavirus Receptor-Dependent and -Independent Mechanisms of Infection.Viruses. 2022 May 24;14(6):1130. doi: 10.3390/v14061130. Viruses. 2022. PMID: 35746603 Free PMC article. Review.
-
Extracellular Vesicles Regulated by Viruses and Antiviral Strategies.Front Cell Dev Biol. 2021 Oct 21;9:722020. doi: 10.3389/fcell.2021.722020. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34746122 Free PMC article. Review.
-
Progressive Multifocal Leukoencephalopathy: Pathogenesis, Diagnostic Tools, and Potential Biomarkers of Response to Therapy.Neurology. 2023 Oct 17;101(16):700-713. doi: 10.1212/WNL.0000000000207622. Epub 2023 Jul 24. Neurology. 2023. PMID: 37487750 Free PMC article. Review.
References
-
- Ferenczy MW, Marshall LJ, Nelson CD, Atwood WJ, Nath A, Khalili K, et al. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 2012;25(3):471–506. 10.1128/CMR.05031-11 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

