The case of eculizumab: litigation and purchases by the Brazilian Ministry of Health
- PMID: 32130309
- PMCID: PMC7017980
- DOI: 10.11606/s1518-8787.2020054001693
The case of eculizumab: litigation and purchases by the Brazilian Ministry of Health
Abstract
Objectives: This study examined the purchases of eculizumab, a high-cost monoclonal antibody used in the treatment of rare diseases by Brazilian federal agencies, in terms of purchased quantities, expenditures, and prices.
Methods: Eculizumab purchases made between March 2007 and December 2018 were analyzed, using secondary data extracted from the Federal Government Purchasing System (SIASG in Portuguese). The following aspects were assessed: number of purchases, purchased quantities, number of daily doses defined per 1,000 inhabitants per year, annual expenditures, and prices. The prices were adjusted by the National Broad Consumer Price Index for December 2018. Linear regression was used for trend analysis.
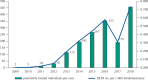
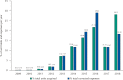
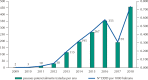
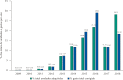
Results: All acquisitions by federal agencies were made by the Brazilian Ministry of Health. The purchases began in 2009 with tender waiver to comply with legal demand. There was an increasing trend in the number of purchases and quantities acquired over time. Two hundred and eighty-three purchases were made, totaling 116,792 units purchased, 28.2% of them in 2018. The adjusted total expenses summed more than R$ 2.44 billion. After market approval by the Brazilian Health Regulatory Agency, the weighted average price fell approximately 35%, to values under the Medicines Market Chamber of Regulation established prices.
Conclusion: Eculizumab represented extremely significant expenditures for the Brazilian Ministry of Health during the period. All purchases were made to meet demands from lawsuits, outside the competitive environment. The market approval of eculizumab promoted an important price reduction. This study indicates the relevance of licensing and the need for permanent monitoring and auditing of drug purchases to meet legal demands.
OBJETIVOS: O estudo examinou as aquisições de eculizumabe, um anticorpo monoclonal de alto custo utilizado no tratamento de doenças raras, pelos órgãos federais brasileiros, em termos das quantidades compradas, gastos e preços.
MÉTODOS: Foram analisadas compras de eculizumabe realizadas entre março de 2007 e dezembro de 2018, por meio de dados secundários extraídos do sistema de compras do governo federal (Siasg). Foram examinados o número de compras, quantidades adquiridas, número de doses diárias definidas por 1.000 habitantes por ano, gastos anuais e preços praticados. Os preços foram corrigidos pelo índice nacional de preços ao consumidor amplo para dezembro de 2018. Regressão linear foi utilizada para análises de tendência.
RESULTADOS: Todas as aquisições por órgãos federais foram realizadas pelo Ministério da Saúde. As compras se iniciaram em 2009, sendo efetuadas por dispensa de licitação e para atendimento de demanda judicial. Houve tendência crescente no número de compras e quantidades adquiridas ao longo do tempo. Foram realizadas 283 compras, totalizando 116.792 unidades adquiridas, 28,2% compradas em 2018. Os gastos totais contratados corrigidos somaram mais de R$ 2,44 bilhões. Após a aprovação do registro pela Agência Nacional de Vigilância Sanitária, o preço médio ponderado caiu aproximadamente 35%, para valores abaixo dos preços estabelecidos pela Câmara de Regulação do Mercado de Medicamentos.
CONCLUSÃO: O eculizumabe representou gastos extremamente significativos para o Ministério da Saúde no período. Todas as compras foram feitas para atendimento de demandas judiciais, fora do ambiente competitivo. Seu registro promoveu queda importante nos preços praticados. O estudo aponta a relevância do registro sanitário e da necessidade de monitoramento e auditoria permanentes das compras de medicamentos para atendimento de demandas judiciais.
Conflict of interest statement
Conflict of Interest: The authors declare no conflict of interest.
Figures


References
-
- 1. Parker C, Omine M, Richards S, Nishimura J, Bessler M, Ware R, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106(12):3699-709. 10.1182/blood-2005-04-1717 - DOI - PMC - PubMed
- Parker C, Omine M, Richards S, Nishimura J, Bessler M, Ware R, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106(12):3699–3709. doi: 10.1182/blood-2005-04-1717. - DOI - PMC - PubMed
-
- 2. Blasco Pelicano M, Rodríguez de Córdoba S, Campistol Plana JM. Síndrome hemolítico urémico atípico. Med Clin (Barc). 2015;145(10):438-45. 10.1016/j.medcli.2014.08.006 - DOI - PubMed
- Blasco Pelicano M, Rodríguez de Córdoba S, Campistol Plana JM. Síndrome hemolítico urémico atípico. Med Clin (Barc) 2015;145(10):438–445. doi: 10.1016/j.medcli.2014.08.006. - DOI - PubMed
-
- 3. Richter T, Nestler-Parr S, Babela R, Khan ZM, Tesoro T, Molsen E, et al. Rare disease terminology and definitions: a systematic global review: report of the ISPOR Rare Disease Special Interest Group. Value Health. 2015;18(6):906-14. 10.1016/j.jval.2015.05.008 - DOI - PubMed
- Richter T, Nestler-Parr S, Babela R, Khan ZM, Tesoro T, Molsen E, et al. Rare disease terminology and definitions: a systematic global review: report of the ISPOR Rare Disease Special Interest Group. Value Health. 2015;18(6):906–914. doi: 10.1016/j.jval.2015.05.008. - DOI - PubMed
-
- 4. Borowitz MJ, Craig FE, DiGiuseppe JA, Illingworth AJ, Rosse W, Sutherland DR, et al. Guidelines for the diagnosis and monitoring of paroxysmal nocturnal hemoglobinuria and related disorders by flow cytometry. Cytometry B Clin Cytom. 2010;78(4):211-30. 10.1002/cyto.b.20525 - DOI - PubMed
- Borowitz MJ, Craig FE, DiGiuseppe JA, Illingworth AJ, Rosse W, Sutherland DR, et al. Guidelines for the diagnosis and monitoring of paroxysmal nocturnal hemoglobinuria and related disorders by flow cytometry. Cytometry B Clin Cytom. 2010;78(4):211–230. doi: 10.1002/cyto.b.20525. - DOI - PubMed
-
- 5. Devos T, Meers S, Boeckx N, Gothot A, Deeren D, Chatelain B, et al. Diagnosis and management of PNH: review and recommendations from a Belgian expert panel. Eur J Haematol. 2018;101(6):737-49. 10.1111/ejh.13166 - DOI - PubMed
- Devos T, Meers S, Boeckx N, Gothot A, Deeren D, Chatelain B, et al. Diagnosis and management of PNH: review and recommendations from a Belgian expert panel. Eur J Haematol. 2018;101(6):737–749. doi: 10.1111/ejh.13166. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

