G1T48, an oral selective estrogen receptor degrader, and the CDK4/6 inhibitor lerociclib inhibit tumor growth in animal models of endocrine-resistant breast cancer
- PMID: 32130619
- PMCID: PMC7103015
- DOI: 10.1007/s10549-020-05575-9
G1T48, an oral selective estrogen receptor degrader, and the CDK4/6 inhibitor lerociclib inhibit tumor growth in animal models of endocrine-resistant breast cancer
Abstract
Purpose: The combination of targeting the CDK4/6 and estrogen receptor (ER) signaling pathways with palbociclib and fulvestrant is a proven therapeutic strategy for the treatment of ER-positive breast cancer. However, the poor physicochemical properties of fulvestrant require monthly intramuscular injections to patients, which limit the pharmacokinetic and pharmacodynamic activity of the compound. Therefore, an orally available compound that more rapidly reaches steady state may lead to a better clinical response in patients. Here, we report the identification of G1T48, a novel orally bioavailable, non-steroidal small molecule antagonist of ER.
Methods: The pharmacological effects and the antineoplastic mechanism of action of G1T48 on tumors was evaluated using human breast cancer cells (in vitro) and xenograft efficacy models (in vivo).
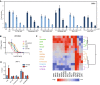
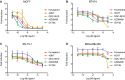
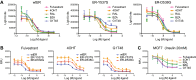
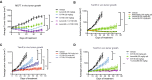
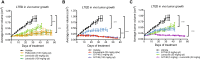
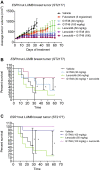
Results: G1T48 is a potent and efficacious inhibitor of estrogen-mediated transcription and proliferation in ER-positive breast cancer cells, similar to the pure antiestrogen fulvestrant. In addition, G1T48 can effectively suppress ER activity in multiple models of endocrine therapy resistance including those harboring ER mutations and growth factor activation. In vivo, G1T48 has robust antitumor activity in a model of estrogen-dependent breast cancer (MCF7) and significantly inhibited the growth of tamoxifen-resistant (TamR), long-term estrogen-deprived (LTED) and patient-derived xenograft tumors with an increased response being observed with the combination of G1T48 and the CDK4/6 inhibitor lerociclib.
Conclusions: These data show that G1T48 has the potential to be an efficacious oral antineoplastic agent in ER-positive breast cancer.
Keywords: CDK4/6 inhibitor; Combination therapies; Endocrine-resistant breast cancer; G1T48; Selective estrogen receptor degrader.
Conflict of interest statement
Jessica A. Sorrentino, Delita A. Thompson, John E. Bisi, and Jay C. Strum are employees of G1 Therapeutics, Inc. Delita A. Thompson, John E. Bisi, Jessica A. Sorrentino, and Jay C. Strum own stock in G1 Therapeutics, Inc. John D. Norris is a paid consultant for G1 Therapeutics, Inc. All other authors have no conflicts of interest.
Figures

References
-
- Rydén L, et al. Aromatase inhibitors alone or sequentially combined with tamoxifen in postmenopausal early breast cancer compared with tamoxifen or placebo - Meta-analyses on efficacy and adverse events based on randomized clinical trials. Breast. 2016;26:106–114. doi: 10.1016/j.breast.2016.01.006. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

