Optimizing the role of androgen deprivation therapy in advanced prostate cancer: Challenges beyond the guidelines
- PMID: 32130741
- PMCID: PMC7154535
- DOI: 10.1002/pros.23967
Optimizing the role of androgen deprivation therapy in advanced prostate cancer: Challenges beyond the guidelines
Abstract
Background: For specific clinical indications, androgen deprivation therapy (ADT) will induce disease prostate cancer (PC) regression, relieve symptoms and prolong survival; however, ADT has a well-described range of side effects, which may have a detrimental effect on the patient's quality of life, necessitating additional interventions or changes in PC treatment. The risk-benefit analysis for initiating ADT in PC patients throughout the PC disease continuum warrants review.
Methods: A 14-member panel comprised of urologic and medical oncologists were chosen for an expert review panel, to provide guidance on a more judicious use of ADT in advanced PC patients. Panel members were chosen based upon their academic and community experience and expertise in the management of PC patients. Four academic members of the panel served as group leaders; the remaining eight panel members were from Large Urology Group Practice Association practices with proven experience in leading their advanced PC clinics. The panel members were assigned to four separate working groups, and were tasked with addressing the role of ADT in specific PC settings.
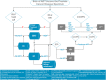
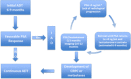
Results: This article describes the practical recommendations of an expert panel for the use of ADT throughout the PC disease continuum, as well as an algorithm summarizing the key recommendations. The target for this publication is all providers (urologists, medical oncologists, radiation oncologists, or advanced practice providers) who evaluate and manage advanced PC patients, regardless of their practice setting.
Conclusion: The panel has provided recommendations for monitoring PC patients while on ADT, recognizing that PC patients will progress despite testosterone suppression and, therefore, early identification of conversion from castrate-sensitive to castration resistance is critical. Also, the requirement to both identify and mitigate side effects of ADT as well as the importance of quality of life maintenance are essential to the optimization of patient care, especially as more combinatorial therapeutic strategies with ADT continue to emerge.
Keywords: androgen deprivation therapy; cancer; consensus; prostate.
© 2020 The Authors. The Prostate published by Wiley Periodicals, Inc.
Conflict of interest statement
Neal D. Shore, MD, FACS, Research/consulting: Amgen, Astellas, AstraZeneca, Bayer, BMS, Dendreon, Ferring, Janssen, Merck, Myovant, Nymox, Pfizer, Sanofi‐Genzyme, and Tolmar; Emmanuel S. Antonarakis, MD, Paid consultant/advisor: Janssen, Pfizer, Sanofi, Dendreon, Bayer, Bristol Myers Squibb, Amgen, Merck, AstraZeneca, Clovis Research grants to his institution: Janssen, Johnson&Johnson, Sanofi, Bristol Myers Squibb, Pfizer, AstraZeneca, Celgene, Merck, Bayer, Clovis Inventor of a biomarker technology that has been licensed to Qiagen; Michael S. Cookson, MD, Advisory board: MDxHealth, Janssen Scientific Affairs, LLC; Bayer Healthcare Pharmaceuticals Inc; Ferring Pharmaceuticals Inc; Consulting agreement: Astellas Pharma US Inc; E. D. Crawford, MD, Consultant advisor: Tolmar, Bayer, MDx, Genomic Health, Janssen, Dendreon, Ferring, Spouse employee: Dendreon Meeting participant or lecturer: Bayer Scientific study or trial: NIH, University of Colorado Cancer Center; Alicia K. Morgans, MD, Honoraria for consulting: Astellas, Sanofi, Bayer, AstraZeneca, Janssen, Genentech Research funding: Bayer; David M. Albala, MD, Speaker: Blue Earth Diagnostics, Genomic Health Advisory board: Cellanyx; Jason Hafron, MD, Scientific study/trial: Janssen Biotech Inc, Pfizer Inc/Astellas Pharma Inc Meeting participant/lecturer: Blue Earth Diagnostics, Janssen Biotech Inc, Pfizer Inc/Astellas Pharma Inc Consultant/Advisor: Dendreon Pharmaceuticals LLC, Janssen Biotech Inc, Pfizer Inc/Astellas Pharma Inc; Richard G. Harris, MD, Advisor/speaker: Jansen, Astellas, Bayer. Advisor Dendreon, and Clovis; Daniel Saltzstein, MD, Speaker/advisor: Janssen and Astellas Pharmaceuticals; Jonathan Henderson, MD, Consultant: Myriad Genetics, Clovis Pharmaceuticals Speaker: Janssen; Benjamin Lowentritt, MD, Speaker and/or consultant: Bayer, Dendreon, Janssen, Astellas, Pfizer, Genomic Health and Myriad; Raoul Concepcion, MD, FACS, Consultant: CUSP, Integra Connect, Clovis, Cellay, Merck, Invitae, Astellas, Janssen, Sun Pharma, Dendreon Speaker's Bureau: Astellas, Pfizer, Amgen, Sun Pharma, Dendreon. The remaining authors declare that there are no conflict of interests.
Figures
References
-
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232‐240. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

