iNOS-inhibitor driven neuroprotection in a porcine retina organ culture model
- PMID: 32130787
- PMCID: PMC7171393
- DOI: 10.1111/jcmm.15091
iNOS-inhibitor driven neuroprotection in a porcine retina organ culture model
Abstract
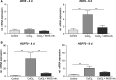
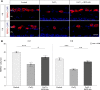
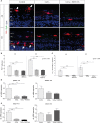
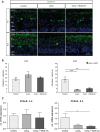
Nitrite oxide plays an important role in the pathogenesis of various retinal diseases, especially when hypoxic processes are involved. This degeneration can be simulated by incubating porcine retinal explants with CoCl2 . Here, the therapeutic potential of iNOS-inhibitor 1400W was evaluated. Degeneration through CoCl2 and treatment with the 1400W were applied simultaneously to porcine retinae explants. Three groups were compared: control, CoCl2 , and CoCl2 + iNOS-inhibitor (1400W). At days 4 and 8, retinal ganglion cells (RGCs), bipolar, and amacrine cells were analysed. Furthermore, the influence on the glia cells and different stress markers were evaluated. Treatment with CoCl2 resulted in a significant loss of RGCs already after 4 days, which was counteracted by the iNOS-inhibitor. Expression of HIF-1α and its downstream targets confirmed the effective treatment with 1400W. After 8 days, the CoCl2 group displayed a significant loss in amacrine cells and also a drastic reduction in bipolar cells was observed, which was prevented by 1400W. The decrease in microglia could not be prevented by the inhibitor. CoCl2 induces strong degeneration in porcine retinae by mimicking hypoxia, damaging certain retinal cell types. Treatment with the iNOS-inhibitor counteracted these effects to some extent, by preventing loss of retinal ganglion and bipolar cells. Hence, this inhibitor seems to be a very promising treatment for retinal diseases.
Keywords: hypoxia; iNOS-inhibitor 1400W; organ culture; retina; retinal ganglion cells.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures


References
-
- Daneshian M, Busquet F, Hartung T, Leist M. Animal use for science in Europe. Altex‐Altern Anim Ex. 2015;32:261‐274. - PubMed
-
- Kuehn S, Hurst J, Jashari A, et al. A novel NMDA triggered porcine organ culture induces retinal ganglion cell apoptosis – chances for replacement of animal experiments. Altern Lab Anim. 2016;44:557‐568. - PubMed
-
- Kuehn S, Hurst J, Rensinghoff F, et al. Degenerative effects of cobalt‐chloride treatment on neurons and microglia in a porcine retina organ culture model. Exp Eye Res. 2017;155:107‐120. - PubMed
-
- Hurst J, Kuehn S, Jashari A, et al. A novel porcine ex vivo retina culture model for oxidative stress induced by H2O2 . Altern Lab Anim. 2017;45:11‐25. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical

