Treatment of Highly Drug-Resistant Pulmonary Tuberculosis
- PMID: 32130813
- PMCID: PMC6955640
- DOI: 10.1056/NEJMoa1901814
Treatment of Highly Drug-Resistant Pulmonary Tuberculosis
Abstract
Background: Patients with highly drug-resistant forms of tuberculosis have limited treatment options and historically have had poor outcomes.
Methods: In an open-label, single-group study in which follow-up is ongoing at three South African sites, we investigated treatment with three oral drugs - bedaquiline, pretomanid, and linezolid - that have bactericidal activity against tuberculosis and to which there is little preexisting resistance. We evaluated the safety and efficacy of the drug combination for 26 weeks in patients with extensively drug-resistant tuberculosis and patients with multidrug-resistant tuberculosis that was not responsive to treatment or for which a second-line regimen had been discontinued because of side effects. The primary end point was the incidence of an unfavorable outcome, defined as treatment failure (bacteriologic or clinical) or relapse during follow-up, which continued until 6 months after the end of treatment. Patients were classified as having a favorable outcome at 6 months if they had resolution of clinical disease, a negative culture status, and had not already been classified as having had an unfavorable outcome. Other efficacy end points and safety were also evaluated.
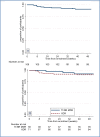
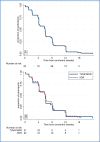
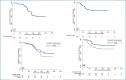
Results: A total of 109 patients were enrolled in the study and were included in the evaluation of efficacy and safety end points. At 6 months after the end of treatment in the intention-to-treat analysis, 11 patients (10%) had an unfavorable outcome and 98 patients (90%; 95% confidence interval, 83 to 95) had a favorable outcome. The 11 unfavorable outcomes were 7 deaths (6 during treatment and 1 from an unknown cause during follow-up), 1 withdrawal of consent during treatment, 2 relapses during follow-up, and 1 loss to follow-up. The expected linezolid toxic effects of peripheral neuropathy (occurring in 81% of patients) and myelosuppression (48%), although common, were manageable, often leading to dose reductions or interruptions in treatment with linezolid.
Conclusions: The combination of bedaquiline, pretomanid, and linezolid led to a favorable outcome at 6 months after the end of therapy in a high percentage of patients with highly drug-resistant forms of tuberculosis; some associated toxic effects were observed. (Funded by the TB Alliance and others; ClinicalTrials.gov number, NCT02333799.).
Copyright © 2020 Massachusetts Medical Society.
Figures
Comment in
-
Triumph and Tragedy of 21st Century Tuberculosis Drug Development.N Engl J Med. 2020 Mar 5;382(10):959-960. doi: 10.1056/NEJMe2000860. N Engl J Med. 2020. PMID: 32130819 No abstract available.
-
Treatment of Highly Drug-Resistant Pulmonary Tuberculosis.N Engl J Med. 2020 Jun 11;382(24):2376. doi: 10.1056/NEJMc2009939. N Engl J Med. 2020. PMID: 32521141 No abstract available.
-
Treatment of Highly Drug-Resistant Pulmonary Tuberculosis.N Engl J Med. 2020 Jun 11;382(24):2376-2377. doi: 10.1056/NEJMc2009939. N Engl J Med. 2020. PMID: 32521142 No abstract available.
References
-
- WHO The End TB Strategy. 2014. [cited 2018 09/09/2018]; Available from: http://www.who.int/tb/strategy/End_TB_Strategy.pdf?ua=1.
-
- Nunn A.J., et al. , A Trial of a Shorter Regimen for Rifampin-Resistant Tuberculosis. N Engl J Med, 2019. 380(13): p. 1201-1213. - PubMed
-
- Gandhi N.R., et al. , Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet, 2006. 368(9547): p. 1575-80. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
