Emergent Elements of Inspiratory Rhythmogenesis: Network Synchronization and Synchrony Propagation
- PMID: 32130872
- PMCID: PMC11221628
- DOI: 10.1016/j.neuron.2020.02.005
Emergent Elements of Inspiratory Rhythmogenesis: Network Synchronization and Synchrony Propagation
Abstract
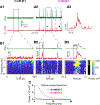
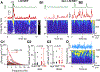
We assessed the mechanism of mammalian breathing rhythmogenesis in the preBötzinger complex (preBötC) in vitro, where experimental tests remain inconsistent with hypotheses of canonical rhythmogenic cellular or synaptic mechanisms, i.e., pacemaker neurons or inhibition. Under rhythmic conditions, in each cycle, an inspiratory burst emerges as (presumptive) preBötC rhythmogenic neurons transition from aperiodic uncorrelated population spike activity to become increasingly synchronized during preinspiration (for ∼50-500 ms), which can trigger inspiratory bursts that propagate to motoneurons. In nonrhythmic conditions, antagonizing GABAA receptors can initiate this synchronization while inducing a higher conductance state in nonrhythmogenic preBötC output neurons. Our analyses uncover salient features of preBötC network dynamics where inspiratory bursts arise when and only when the preBötC rhythmogenic subpopulation strongly synchronizes to drive output neurons. Furthermore, downstream propagation of preBötC network activity, ultimately to motoneurons, is dependent on the strength of input synchrony onto preBötC output neurons exemplifying synchronous propagation of network activity.
Keywords: breathing; central pattern generator; network dynamics; preBötzinger complex; rhythm; synaptic correlation; synchrony; synfire chain.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures





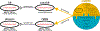
References
-
- Barlow HB (1961). Possible principles underlying the transformation of sensory messages. In Sensory communication, Rosenblith WA, ed. (Cambridge, MA: The MIT Press; ), pp. 217–234.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

