Restoring Cellular Energetics Promotes Axonal Regeneration and Functional Recovery after Spinal Cord Injury
- PMID: 32130884
- PMCID: PMC7188478
- DOI: 10.1016/j.cmet.2020.02.002
Restoring Cellular Energetics Promotes Axonal Regeneration and Functional Recovery after Spinal Cord Injury
Abstract
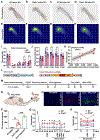
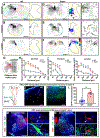
Axonal regeneration in the central nervous system (CNS) is a highly energy-demanding process. Extrinsic insults and intrinsic restrictions lead to an energy crisis in injured axons, raising the question of whether recovering energy deficits facilitates regeneration. Here, we reveal that enhancing axonal mitochondrial transport by deleting syntaphilin (Snph) recovers injury-induced mitochondrial depolarization. Using three CNS injury mouse models, we demonstrate that Snph-/- mice display enhanced corticospinal tract (CST) regeneration passing through a spinal cord lesion, accelerated regrowth of monoaminergic axons across a transection gap, and increased compensatory sprouting of uninjured CST. Notably, regenerated CST axons form functional synapses and promote motor functional recovery. Administration of the bioenergetic compound creatine boosts CST regenerative capacity in Snph-/- mice. Our study provides mechanistic insights into intrinsic regeneration failure in CNS and suggests that enhancing mitochondrial transport and cellular energetics are promising strategies to promote regeneration and functional restoration after CNS injuries.
Keywords: CNS injury; axon regeneration; axonal transport; creatine; energy deficits; energy metabolism; mitochondria; spinal cord injury; syntaphilin.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Interests The authors declare no competing financial interests.
Figures





References
-
- Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, and Fehlings MG (2017). Traumatic spinal cord injury. Nature Reviews Disease Primers 3, 17018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

