Microglial Homeostasis Requires Balanced CSF-1/CSF-2 Receptor Signaling
- PMID: 32130903
- PMCID: PMC7370656
- DOI: 10.1016/j.celrep.2020.02.028
Microglial Homeostasis Requires Balanced CSF-1/CSF-2 Receptor Signaling
Abstract
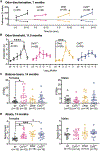
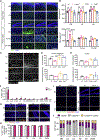
CSF-1R haploinsufficiency causes adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP). Previous studies in the Csf1r+/- mouse model of ALSP hypothesized a central role of elevated cerebral Csf2 expression. Here, we show that monoallelic deletion of Csf2 rescues most behavioral deficits and histopathological changes in Csf1r+/- mice by preventing microgliosis and eliminating most microglial transcriptomic alterations, including those indicative of oxidative stress and demyelination. We also show elevation of Csf2 transcripts and of several CSF-2 downstream targets in the brains of ALSP patients, demonstrating that the mechanisms identified in the mouse model are functional in humans. Our data provide insights into the mechanisms underlying ALSP. Because increased CSF2 levels and decreased microglial Csf1r expression have also been reported in Alzheimer's disease and multiple sclerosis, we suggest that the unbalanced CSF-1R/CSF-2 signaling we describe in the present study may contribute to the pathogenesis of other neurodegenerative conditions.
Keywords: ALSP; CSF-1R; GM-CSF; demyelination; leukodystrophy; microglia; neurodegeneration.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures
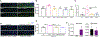




References
-
- Baldwin GC, Benveniste EN, Chung GY, Gasson JC, and Golde DW (1993). Identification and characterization of a high-affinity granulocyte-macrophage colony-stimulating factor receptor on primary rat oligodendrocytes. Blood 82, 3279–3282. - PubMed
-
- Berrebi D, Bruscoli S, Cohen N, Foussat A, Migliorati G, Bouchet-Delbos L, Maillot MC, Portier A, Couderc J, Galanaud P, et al. (2003). Synthesis of glucocorticoid-induced leucine zipper (GILZ) by macrophages: an anti-inflammatory and immunosuppressive mechanism shared by glucocorticoids and IL-10. Blood 101, 729–738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

