Microcircuit Rules Governing Impact of Single Interneurons on Purkinje Cell Output In Vivo
- PMID: 32130904
- PMCID: PMC7059114
- DOI: 10.1016/j.celrep.2020.02.009
Microcircuit Rules Governing Impact of Single Interneurons on Purkinje Cell Output In Vivo
Abstract
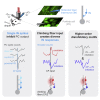
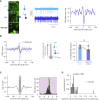
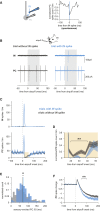
The functional impact of single interneurons on neuronal output in vivo and how interneurons are recruited by physiological activity patterns remain poorly understood. In the cerebellar cortex, molecular layer interneurons and their targets, Purkinje cells, receive excitatory inputs from granule cells and climbing fibers. Using dual patch-clamp recordings from interneurons and Purkinje cells in vivo, we probe the spatiotemporal interactions between these circuit elements. We show that single interneuron spikes can potently inhibit Purkinje cell output, depending on interneuron location. Climbing fiber input activates many interneurons via glutamate spillover but results in inhibition of those interneurons that inhibit the same Purkinje cell receiving the climbing fiber input, forming a disinhibitory motif. These interneuron circuits are engaged during sensory processing, creating diverse pathway-specific response functions. These findings demonstrate how the powerful effect of single interneurons on Purkinje cell output can be sculpted by various interneuron circuit motifs to diversify cerebellar computations.
Keywords: Purkinje cell; cerebellum; climbing fiber; glutamate spillover; in vivo; inhibition; interneuron; patch clamp; synaptic integration; two-photon imaging.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures





Similar articles
-
Efficient generation of reciprocal signals by inhibition.J Neurophysiol. 2012 May;107(9):2453-62. doi: 10.1152/jn.00083.2012. Epub 2012 Feb 1. J Neurophysiol. 2012. PMID: 22298833 Free PMC article.
-
Time-invariant feed-forward inhibition of Purkinje cells in the cerebellar cortex in vivo.J Physiol. 2016 May 15;594(10):2729-49. doi: 10.1113/JP271518. Epub 2016 Apr 10. J Physiol. 2016. PMID: 26918702 Free PMC article.
-
The role of interneurons in shaping Purkinje cell responses in the cerebellar cortex.J Neurosci. 2011 Jul 20;31(29):10463-73. doi: 10.1523/JNEUROSCI.1350-11.2011. J Neurosci. 2011. PMID: 21775592 Free PMC article.
-
Climbing fibers mediate vestibular modulation of both "complex" and "simple spikes" in Purkinje cells.Cerebellum. 2015 Oct;14(5):597-612. doi: 10.1007/s12311-015-0725-1. Cerebellum. 2015. PMID: 26424151 Review.
-
Dendritic calcium signaling in cerebellar Purkinje cell.Neural Netw. 2013 Nov;47:11-7. doi: 10.1016/j.neunet.2012.08.001. Epub 2012 Sep 5. Neural Netw. 2013. PMID: 22985934 Review.
Cited by
-
In Vivo Whole-Cell Patch-Clamp Methods: Recent Technical Progress and Future Perspectives.Sensors (Basel). 2021 Feb 19;21(4):1448. doi: 10.3390/s21041448. Sensors (Basel). 2021. PMID: 33669656 Free PMC article. Review.
-
Developmental emergence of two-stage nonlinear synaptic integration in cerebellar interneurons.Elife. 2021 Nov 3;10:e65954. doi: 10.7554/eLife.65954. Elife. 2021. PMID: 34730085 Free PMC article.
-
Heterogeneous encoding of temporal stimuli in the cerebellar cortex.Nat Commun. 2023 Nov 21;14(1):7581. doi: 10.1038/s41467-023-43139-9. Nat Commun. 2023. PMID: 37989740 Free PMC article.
-
Transcriptome programs involved in the development and structure of the cerebellum.Cell Mol Life Sci. 2021 Oct;78(19-20):6431-6451. doi: 10.1007/s00018-021-03911-w. Epub 2021 Aug 18. Cell Mol Life Sci. 2021. PMID: 34406416 Free PMC article. Review.
-
Afferent convergence to a shared population of interneuron AMPA receptors.Nat Commun. 2023 May 30;14(1):3113. doi: 10.1038/s41467-023-38854-2. Nat Commun. 2023. PMID: 37253743 Free PMC article.
References
-
- Albus J.S. A theory of cerebellar function. Math. Biosci. 1971;10:25–61.
-
- Asztely F., Erdemli G., Kullmann D.M. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18:281–293. - PubMed
-
- Auger C., Attwell D. Fast removal of synaptic glutamate by postsynaptic transporters. Neuron. 2000;28:547–558. - PubMed
-
- Badura A., Schonewille M., Voges K., Galliano E., Renier N., Gao Z., Witter L., Hoebeek F.E., Chédotal A., De Zeeuw C.I. Climbing fiber input shapes reciprocity of Purkinje cell firing. Neuron. 2013;78:700–713. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

