Inhibitory Effect and Mechanism of Action of Quercetin and Quercetin Diels-Alder anti-Dimer on Erastin-Induced Ferroptosis in Bone Marrow-Derived Mesenchymal Stem Cells
- PMID: 32131401
- PMCID: PMC7139729
- DOI: 10.3390/antiox9030205
Inhibitory Effect and Mechanism of Action of Quercetin and Quercetin Diels-Alder anti-Dimer on Erastin-Induced Ferroptosis in Bone Marrow-Derived Mesenchymal Stem Cells
Abstract
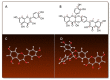
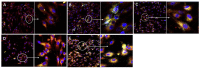
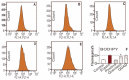
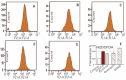
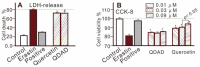
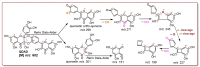
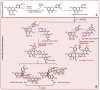
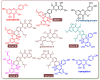
In this study, the anti-ferroptosis effects of catecholic flavonol quercetin and its metabolite quercetin Diels-Alder anti-dimer (QDAD) were studied using an erastin-treated bone marrow-derived mesenchymal stem cell (bmMSCs) model. Quercetin exhibited higher anti-ferroptosis levels than QDAD, as indicated by 4,4-difluoro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-undecanoic acid (C11-BODIPY), 2',7'-dichlorodihydrofluoroscein diacetate (H2DCFDA), lactate dehydrogenase (LDH) release, cell counting kit-8 (CCK-8), and flow cytometric assays. To understand the possible pathways involved, the reaction product of quercetin with the 1,1-diphenyl-2-picrylhydrazyl radical (DPPH●) was measured using ultra-performance liquid-chromatography coupled with electrospray-ionization quadrupole time-of-flight tandem mass spectrometry (UHPLC-ESI-Q-TOF-MS). Quercetin was found to produce the same clusters of molecular ion peaks and fragments as standard QDAD. Furthermore, the antioxidant effects of quercetin and QDAD were compared by determining their 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide radical-scavenging, Cu2+-reducing, Fe3+-reducing, lipid peroxidation-scavenging, and DPPH●-scavenging activities. Quercetin consistently showed lower IC50 values than QDAD. These findings indicate that quercetin and QDAD can protect bmMSCs from erastin-induced ferroptosis, possibly through the antioxidant pathway. The antioxidant pathway can convert quercetin into QDAD-an inferior ferroptosis-inhibitor and antioxidant. The weakening has highlighted a rule for predicting the relative anti-ferroptosis and antioxidant effects of catecholic flavonols and their Diels-Alder dimer metabolites.
Keywords: Diels-Alder dimer; QDAD; anti-ferroptosis; antioxidant; erastin; quercetin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Structure-activity relationship and mechanism of four monostilbenes with respect to ferroptosis inhibition.RSC Adv. 2020 Aug 21;10(52):31171-31179. doi: 10.1039/d0ra04896h. eCollection 2020 Aug 21. RSC Adv. 2020. PMID: 35520676 Free PMC article.
-
Simultaneous Study of Anti-Ferroptosis and Antioxidant Mechanisms of Butein and (S)-Butin.Molecules. 2020 Feb 5;25(3):674. doi: 10.3390/molecules25030674. Molecules. 2020. PMID: 32033283 Free PMC article.
-
Antioxidant and Cytoprotective effects of Pyrola decorata H. Andres and its five phenolic components.BMC Complement Altern Med. 2019 Oct 21;19(1):275. doi: 10.1186/s12906-019-2698-y. BMC Complement Altern Med. 2019. PMID: 31638966 Free PMC article.
-
Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis.Curr Top Microbiol Immunol. 2017;403:143-170. doi: 10.1007/82_2016_508. Curr Top Microbiol Immunol. 2017. PMID: 28204974 Review.
-
The Role of Erastin in Ferroptosis and Its Prospects in Cancer Therapy.Onco Targets Ther. 2020 Jun 11;13:5429-5441. doi: 10.2147/OTT.S254995. eCollection 2020. Onco Targets Ther. 2020. PMID: 32606760 Free PMC article. Review.
Cited by
-
Structure-activity relationship and mechanism of four monostilbenes with respect to ferroptosis inhibition.RSC Adv. 2020 Aug 21;10(52):31171-31179. doi: 10.1039/d0ra04896h. eCollection 2020 Aug 21. RSC Adv. 2020. PMID: 35520676 Free PMC article.
-
Crosstalk between ferroptosis and chondrocytes in osteoarthritis: a systematic review of in vivo and in vitro studies.Front Immunol. 2023 Jul 14;14:1202436. doi: 10.3389/fimmu.2023.1202436. eCollection 2023. Front Immunol. 2023. PMID: 37520558 Free PMC article.
-
Emerging Ferroptosis Involvement in Amyotrophic Lateral Sclerosis Pathogenesis: Neuroprotective Activity of Polyphenols.Molecules. 2025 Mar 8;30(6):1211. doi: 10.3390/molecules30061211. Molecules. 2025. PMID: 40141987 Free PMC article. Review.
-
Antioxidant mechanisms and products of four 4',5,7-trihydroxyflavonoids with different structural types.RSC Med Chem. 2022 Nov 3;14(1):173-182. doi: 10.1039/d2md00333c. eCollection 2023 Jan 25. RSC Med Chem. 2022. PMID: 36760741 Free PMC article.
-
Scandium Ion-Promoted Electron-Transfer Disproportionation of 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-Oxide (PTIO•) in Acetonitrile and Its Regeneration Induced by Water.Int J Mol Sci. 2024 Apr 17;25(8):4417. doi: 10.3390/ijms25084417. Int J Mol Sci. 2024. PMID: 38674002 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

