Vaccination Against Amyloidogenic Aggregates in Pancreatic Islets Prevents Development of Type 2 Diabetes Mellitus
- PMID: 32131431
- PMCID: PMC7157615
- DOI: 10.3390/vaccines8010116
Vaccination Against Amyloidogenic Aggregates in Pancreatic Islets Prevents Development of Type 2 Diabetes Mellitus
Abstract
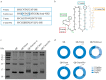
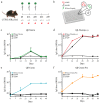
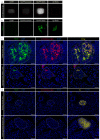
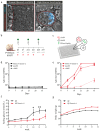
Type 2 diabetes mellitus (T2DM) is a chronic progressive disease characterized by insulin resistance and insufficient insulin secretion to maintain normoglycemia. The majority of T2DM patients bear amyloid deposits mainly composed of islet amyloid polypeptide (IAPP) in their pancreatic islets. These-originally β-cell secretory products-extracellular aggregates are cytotoxic for insulin-producing β-cells and are associated with β-cell loss and inflammation in T2DM advanced stages. Due to the absence of T2DM preventive medicaments and the presence of only symptomatic drugs acting towards increasing hormone secretion and action, we aimed at establishing a novel disease-modifying therapy targeting the cytotoxic IAPP deposits in order to prevent the development of T2DM. We generated a vaccine based on virus-like particles (VLPs), devoid of genomic material, coupled to IAPP peptides inducing specific antibodies against aggregated, but not monomeric IAPP. Using a mouse model of islet amyloidosis, we demonstrate in vivo that our vaccine induced a potent antibody response against aggregated, but not soluble IAPP, strikingly preventing IAPP depositions, delaying onset of hyperglycemia and the induction of the associated pro-inflammatory cytokine Interleukin 1β (IL-1β). We offer the first cost-effective and safe disease-modifying approach targeting islet dysfunction in T2DM, preventing pathogenic aggregates without disturbing physiological IAPP function.
Keywords: T2DM; amyloid; islet amyloid polypeptide; vaccine; virus-like particle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Anti-IAPP Monoclonal Antibody Improves Clinical Symptoms in a Mouse Model of Type 2 Diabetes.Vaccines (Basel). 2021 Nov 12;9(11):1316. doi: 10.3390/vaccines9111316. Vaccines (Basel). 2021. PMID: 34835247 Free PMC article.
-
Islet amyloid and type 2 diabetes: from molecular misfolding to islet pathophysiology.Biochim Biophys Acta. 2001 Nov 29;1537(3):179-203. doi: 10.1016/s0925-4439(01)00078-3. Biochim Biophys Acta. 2001. PMID: 11731221 Review.
-
Differential Activation of Innate Immune Pathways by Distinct Islet Amyloid Polypeptide (IAPP) Aggregates.J Biol Chem. 2016 Apr 22;291(17):8908-17. doi: 10.1074/jbc.M115.712455. Epub 2016 Jan 19. J Biol Chem. 2016. PMID: 26786104 Free PMC article.
-
Causative factors for formation of toxic islet amyloid polypeptide oligomer in type 2 diabetes mellitus.Clin Interv Aging. 2015 Nov 19;10:1873-9. doi: 10.2147/CIA.S95297. eCollection 2015. Clin Interv Aging. 2015. PMID: 26604727 Free PMC article. Review.
-
The human serum protein C4b-binding protein inhibits pancreatic IAPP-induced inflammasome activation.Diabetologia. 2017 Aug;60(8):1522-1533. doi: 10.1007/s00125-017-4286-3. Epub 2017 May 12. Diabetologia. 2017. PMID: 28500395 Free PMC article.
Cited by
-
β-Cell Death in Diabetes: Past Discoveries, Present Understanding, and Potential Future Advances.Metabolites. 2021 Nov 22;11(11):796. doi: 10.3390/metabo11110796. Metabolites. 2021. PMID: 34822454 Free PMC article. Review.
-
Genomic Medicine and Advances in Vaccine Technology and Development in the Developing and Developed World.Vaccines (Basel). 2020 Dec 24;9(1):9. doi: 10.3390/vaccines9010009. Vaccines (Basel). 2020. PMID: 33374343 Free PMC article.
-
Amylin: emergent therapeutic opportunities in overweight, obesity and diabetes mellitus.Nat Rev Endocrinol. 2025 Aug;21(8):482-494. doi: 10.1038/s41574-025-01125-9. Epub 2025 May 13. Nat Rev Endocrinol. 2025. PMID: 40360789 Review.
-
A VLPs based vaccine protects against Zika virus infection and prevents cerebral and testicular damage.NPJ Vaccines. 2025 May 27;10(1):107. doi: 10.1038/s41541-025-01163-4. NPJ Vaccines. 2025. PMID: 40425591 Free PMC article.
-
Okinawa-Based Nordic Diet Decreases Plasma Levels of IAPP and IgA against IAPP Oligomers in Type 2 Diabetes Patients.Int J Mol Sci. 2024 Jul 12;25(14):7665. doi: 10.3390/ijms25147665. Int J Mol Sci. 2024. PMID: 39062913 Free PMC article.
References
-
- Kahn B.B. Type 2 Diabetes: When Insulin Minireview Secretion Fails to Compensate for Insulin Resistance docking proteins called insulin receptor substrates (IRSs). IRSs bind to various effector molecules including the regulatory subunit of phosphoinositol 3-kinase. Cell. 1998;92:593–596. doi: 10.1016/S0092-8674(00)81125-3. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

